
Navitus senior vice president and chief pharmacy officer Brent Eberle talks about how partnering with CivicaScript fits into its cost-plus model.

Navitus senior vice president and chief pharmacy officer Brent Eberle talks about how partnering with CivicaScript fits into its cost-plus model.
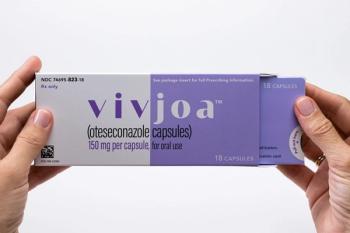
Vivjoa, approved by the FDA in April 2022, is an oral therapy and the first for chronic yeast infections.

Some insulins, as well as some drugs used in emergency care, will now be offered at $0 copay for eligible patients.

Investigators suggest that policies that improve price transparency and increase competition for generic drugs could prevent patients and Medicare from overpaying on generic drugs.

Drugs approved with an FDA breakthrough designation can provide value that offsets their higher costs, finds study conducted by Tufts Center for the Evaluation of Value and Risk in Health.

In 2023, a wave of biosimilar launches is expected, including seven biosimilars of Humira.

In the future, payers plan to leverage reinsurance and value- or outcomes-based contracting to provide access to high-cost therapies.
Blue Cross Blue Shield of North Carolina found that uptake of Trikafta was rapid after approval and total cost of care increased 52% despite reductions in hospitalizations and nonpharmacy costs.

Under a new add-on program, plan members will have access to an expanded list of FDA-approved specialty generic medications with no copays.

The removal of prior authorizations for buprenorphine for use in opioid use disorder was associated with a statistically significant increase in the number of prescriptions filled among Medicaid populations in Illinois but not in California, which had already been seeing an increase in use of such therapies.

Effective July 1, 2022, CVS Caremark has removed 16 drugs and added 14 to its Standard Control Formulary.
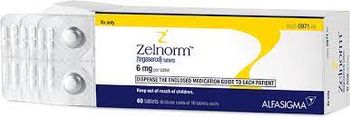
Zelnorm is used to treat adult women less than 65 years of age with irritable bowel syndrome with constipation.
Sanofi has lowered the out-of-pocket cost for those without insurance of its insulins to $35 for a 30-day supply through the company’s savings program.

Diacomit was approved in 2018 for seizures associated with Dravet syndrome, a rare pediatric disorder.

Benefits leaders surveyed in PSG’s Trends in Drug Benefit Design Report say the importance of the member experience has increased since the start of the pandemic.
Xipere became commercially available in March 2022. It is the first therapy for patients with macular edema that provides a targeted delivery to the retina.
A study in the journal Blood has found that the cost-effectiveness of the Polivy treatment regimen used to treat patients with diffuse large B-cell lymphoma would decrease if the five-year progression-free survival decreased.

The Federal Trade Commission said that paying or accepting rebates or fees in exchange for excluding lower cost drugs on formularies could be considered commercial bribery.
A literature review by AmerisourceBergen's Xcenda identifies a link between high cost sharing and patient nonadherence to medication regimens.

Joe Murad, president and CEO of the pharmacy benefit manager WithMe Health, talks about how consumer-driven healthcare requires access, transparency, and easier navigation.

Researchers writing in Health Affairs have found that new patents for updated devices have delayed generic competition in asthma and COPD treatments.

A generic is available for one, and Centers for Medicare & Medicaid Services has removed two others from Medicare Part D coverage.

Two studies conducted by AllianceRx Walgreens Prime call attention to the importance of adherence for reducing risk of negative health outcomes and financial costs.

In some cases, the exclusions favored brand medicines that were more expensive than the excluded generics.

This trend in prescription drug pricing outpaces growth in prices for other healthcare services.