Pfizer’s biologics license application (BLA) for the gene therapy fidanacogene elaparvovec has been assigned a PDUFA date in the second quarter of 2024.

Pfizer’s biologics license application (BLA) for the gene therapy fidanacogene elaparvovec has been assigned a PDUFA date in the second quarter of 2024.
Blincyto is immuno-oncology therapy that targets CD19 surface antigens on B cells to treat patients with acute lymphoblastic leukemia. Its current wholesale acquisition cost price is $4,900.15 per vial.

Now with the name Litfulo, ritlecitinib is the first oral treatment for adolescents with alopecia. It has an annual list price of $49,000.

Elevidys is a one-time therapy that delivers to muscle a gene that codes for a shortened, functional form of dystrophin, which is mutated in Duchenne muscular dystrophy.
The company plans to discontinue further research of obeticholic acid to treat nonalcoholic steatohepatitis (NASH) after the FDA asked for long-term outcomes data.

Regulators indicated there was a lack of evidence of effectiveness and lack of clinical trials done to support the application.

Vyvgart Hytrulo’s under-the-skin administration means it can be given in 30 to 90 seconds, compared with one hour for the intravenous product. It will be priced at parity to Vyvgart, which has a net price of $225,000 annually.

Jardiance and Synjardy are the first SGLT2 inhibitors approved for children with type 2 diabetes.

Imetelstat targets telomerase to inhibit uncontrolled proliferation of malignant stem and progenitor cells in myeloid hematologic malignancies.

Lodoco targets the inflammation that is an underlying cause of atherosclerotic cardiovascular disease. It will be available in the second half of 2023.
The FDA is requesting additional data and analysis for olorofim. F2G plans to resubmit revised NDA with complete data set from phase 2 study.
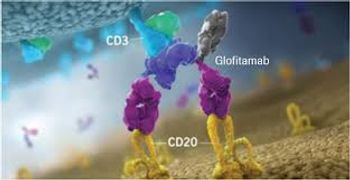
Columvi will cost about $350,000 for a fixed duration therapy with 12 treatments over eight and a half months.

Linzess is first FDA-approved prescription therapy for functional constipation in children 6 to 17 years of age.
If approved, nirsevimab would be the first immunization specifically to protect infants through their first RSV season. The Prescription Drug User Fee Act date is in the third quarter of 2023.
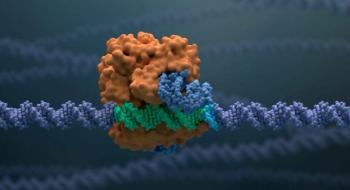
Exagamglogene autotemcel is being evaluated for patients with sickle cell disease and beta thalassemia, in which a patient’s own hematopoietic stem cells are edited to produce high levels of fetal hemoglobin in red blood cells.

Vevye (previously CyclASol) is a water-free, preservative-free solution, which allows for improved bioavailability and better efficacy on the target tissue.
The FDA has assigned an action date of Sept. 23, 2023. If approved, this would be the first frontline treatment advancement in decades for patients with advanced or recurrent endometrial cancer.

Prevymis was approved to prevent CMV infection in adults after an allogeneic hematopoietic stem cell transplant in 2017.

The combination of Lynparza and abiraterone reduced the risk of disease progression or death by 76% vs. abiraterone alone in patients with BRCA-mutated metastatic castration-resistant prostate cancer.
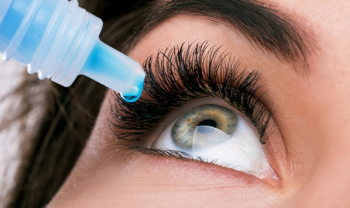
Miebo is the first and only prescription eye drop approved for dry eye disease (DED) that directly targets tear evaporation.
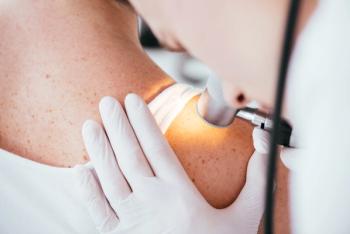
Lifileucel is a polyclonal tumor infiltrating lymphocyte (TIL) therapy designed for patients with advanced melanoma who have experienced progression after previous treatment with anti-PD-1/L1 therapy and targeted therapy.

The frequency of drug manufacturer coupon utilization is associated with more market competition—but not patients’ out-of-pocket costs.

More than 11.6 million treatment courses of the medication have been prescribed in the U.S. to date.

The FDA recently approved Brixadi to treat moderate to severe opioid use disorder in patients who have already started treatment with a transmucosal form of buprenorphine, and have approved Opvee as an emergency treatment to reverse known or suspected opioid overdose in people ages 12 years and older.

The list price for a 30-day supply of the drug, Rinvoq, is around $6,125. Eligible patients may be able to benefit from copay savings cards and other financial support programs from AbbVie.