The FDA has set a Prescription Drug User Fee Act date of Aug. 10, 2024, for Human Acellular Vessel to repair arteries in patients with extremity vascular trauma.

The FDA has set a Prescription Drug User Fee Act date of Aug. 10, 2024, for Human Acellular Vessel to repair arteries in patients with extremity vascular trauma.
The FDA had issued a complete response letter (CRL) in July 2023 for IPX203 and asked for additional pharmacokinetic data of carbidopa, one of the drugs the oral, extended release therapy.
The Prescription Drug User Fee Act date is June 7, 2024, for the additional indication of preventing respiratory syncytial virus (RSV) in adults aged 50 to 59 who are at increased risk. It is already approved for those over the age of 60.
The FDA has set an action date of Nov. 29, 2024, to review acoramidis to treat patients with transthyretin amyloid cardiomyopathy.

The Oncologic Drugs Advisory Committee will meet on March 15, 2024, to review overall survival data for Abecma in earlier lines of treatment in relapsed or refractory multiple myeloma.

Defender Pharmaceuticals is working with U.S. Naval Medical Research Unit and NASA to develop intranasal scopolamine for use in military personnel and astronauts.
Bristol Myers Squibb is seeking approval of Breyanzi, a CD19-directed CAR T-cell therapy, to treat patients with follicular lymphoma and mantle cell lymphoma.
Gammagard is an intravenous immunoglobulin therapy now approved to improve neuromuscular disability and impairment in adults with chronic inflammatory demyelinating polyneuropathy.

Celltrion’s application is based on data from a phase 3 trial of patients with rheumatoid arthritis comparing its biosimilar with Actemra.
Eosinophilic esophagitis is progressive disease driven in part by type 2 inflammation. Dupixent is the first treatment for children as young as 1 year old.
Balversa targets FGFR3 genetic alterations and is approved as a second-line treatment for in adult patients with metastatic urothelial carcinoma.
Last year saw the approval of several firsts, including the first vaccines to prevent respiratory syncytial virus in older adults and infants and a first vaccine to prevent the mosquito-borne virus chikungunya.

HyQvia is now available a maintenance therapy for adults with chronic inflammatory demyelinating polyneuropathy, which can lead to weakness and loss of feeling in the arms and legs.
With a cost of $2.2 million, Casgevy is a CRISPR/Cas9 gene-edited cell therapy that is now also approved to treat beta thalassemia. It was approved to treat sickle cell disease in December 2023.
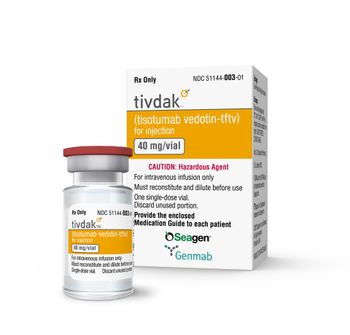
Tivdak was granted accelerated approval in September 2021 to treat patients with recurrent or metastatic cervical cancer. The goal date for full approval is May 9, 2024.
The FDA cited issues with a third-party manufacturing company. Zolbetuximab is being reviewed to treat patients with stomach cancer.
Zelsuvmi, a first-in-class topical treatment approved for patients with molluscum contagiosum, will be available in the second half of 2024.

If approved, Accord’s biosimilar would treat several autoimmune conditions and would be launched no later than May 15, 2025
Loqtorzi has a wholesale acquisition cost of $8,892.03 per single-use vial.


Wainua is approved to treat patients with hereditary transthyretin-mediated amyloid polyneuropathy and can be self-administered via an auto-injector.

This is the first treatment for patients with primary immunoglobulin A nephropathy to be granted full approval.

This is the second complete response letter for gefapixant, and FDA officials said the application did not provide evidence of effectiveness.
Outlook Therapeutics plans to begin a study in the first quarter of 2024 to address the issues identified in a FDA complete response letter. If approved, Lytenava would be the only bevacizumab product to specifically treat age-related macular degeneration.

In other generic news, the FDA has approved generics of the osteoporosis drug Forteo and Amneal is developing a generic version of Vascepa.