A phase 3 trials showed Tivdak demonstrated a 30% reduction in the risk of death. The U.S. list price is $6,599 per 40 mg single dose vial.

A phase 3 trials showed Tivdak demonstrated a 30% reduction in the risk of death. The U.S. list price is $6,599 per 40 mg single dose vial.

Xolremdi is the first therapy for WHIM syndrome, which can cause recurrent lung infections and papillomavirus-related warts. It’s available in two doses: 400 mg for an annual cost of $496,400 and 300 mg for an annual cost of $372,300.

Libervant is film version of diazepam that is placed inside the cheek to treat children between 2 and 5 years of age who have refractory epilepsy.

Beqvez (fidanacogene elaparvovec) is priced at $3.5 million, which is on parity with Hemgenix, the first one-time therapy to treat adults with hemophilia B. Pfizer’s warranty will refund insurers and continue to provide coverage for patients if they change insurers.

Ojemda will have a wholesale acquisition cost of $33,916 for a 28 day supply and will be distributed through specialty pharmacies Biologics by McKesson and Onco360.

Lutathera is a radiotherapeutic that is now approved for patients 12 years and older neuroendocrine tumors in the pancreas or gastrointestinal tract.
ImmunityBio’s Anktiva is an antibody cytokine fusion protein approved to treat patients with non-muscle invasive bladder cancer.

Prademagene zamikeracel is a cell therapy designed to incorporate the functional collagen-producing COL7A1 gene into a patient’s own skin cells. The FDA is asking for additional information on manufacturing practices.
Entyvio as an injection and subcutaneous formulations is approved to treat both Crohn’s and ulcerative colitis.

Alvotech’s Selarsdi (ustekinumab-aekn), a biosimilar referencing Stelara (ustekinumab), gained FDA approval, making it the second ustekinumab biosimilar and second for the company to be given the green light for the American market.

The vaccine combines the antigenic components of its two meningococcal vaccines: Bexsero and Menveo. The action date is Feb. 14, 2025.
Fasenra is now approved to treat patients aged 6 and older with eosinophilic asthma, a rare form of asthma.
The FDA is reviewing two novel therapies: a psychedelic-assisted therapy for PTSD with a target action date of Aug. 11, 2024, and therapy for schizophrenia that does not directly block dopamine receptors with an action date of Sept. 26, 2024.
The FDA is currently reviewing tarlatamab for small-cell lung cancer with a review date in June and datopotamab deruxtecan for non-small cell lung cancer with a review date in the fourth quarter.

FDA officials are looking for additional information about the infusion device used to deliver apomorphine.

The FDA is also considering a separate BLA for a 2 mL syringe. Bimzelx is already available to treat plaque psoriasis as a 1 ml syringe; a dose is two subcutaneous injections with a cost of $7,200 per syringe.
Zevtera was approved to treat Staphylococcus aureus bloodstream infections, acute bacterial skin and skin structure infections; and community-acquired bacterial pneumonia.

Fanapt is now approved to treat adults acute manic or mixed episodes associated with bipolar I disorder. It is also available to treat schizophrenia.
Voydeya was approved for patients with paroxysmal nocturnal hemoglobinuria rare, progressive blood disorder that results in the developed of abnormal blood cells that are missing important proteins.

Vemlidy is now approved to treat patients 6 years and older with chronic hepatitis B virus infection.
Vafseo, an oral therapy to treat patients with anemia due to chronic kidney, will be available in January 2025. About 40% of patients who could be treated with Vafseo have coverage through Medicare Advantage plans.
Sotatercept — now with the brand name Winrevair — will be available through specialty pharmacies by the end of April. The price of Winrevair is $14,000 per vial.
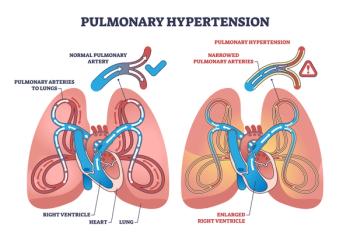
J&J’s Opsynvi is single-tablet combination of macitentan, an endothelin receptor antagonist, and tadalafil, a PDE5 inhibitor. It will be priced on parity with Opsumit, which is also a J&J product to treat patients with pulmonary arterial hypertension.
FDA officials indicated that the confirmatory trial for odronextamab for patients with follicular lymphoma and diffuse large B-cell lymphoma should be under way before resubmission.
The full approval was based on the confirmatory phase 3 MIRASOL trial, which showed that Elahere resulted in a 33% reduction in risk of death and a 35% reduction in the risk of cancer progression.