
Agamree is a novel corticosteroid that will be available in the first quarter of 2024.

Agamree is a novel corticosteroid that will be available in the first quarter of 2024.

Mirikizumab — now with the brand name — Omvoh has a list price of $9,593 per month for the IV formulation and $10,360 per subcutaneous administration.
N-803 — with the brand name Anktiva — plus the Bacillus Calmette-Guérin vaccine is being reviewed by the FDA to treat non-muscle-invasive bladder cancer. The goal date is April 23, 2023.
Akebia has resubmitted its NDA for vadadustat with postmarketing safety data from Japan, where it has been on the market for more than three years. The new review date is March 27, 2024.
Tibsovo is also approved to treat patients with IDH1-mutant acute myeloid leukemia and bile duct cancer.
Byooviz was approved as the first biosimilar to Lucentis and launched with a list price of $1,130 per single-use vial.
The FDA is requesting additional efficacy data to support an approval for patients with chronic spontaneous urticaria; results of an ongoing study are expected late next year.
Zymfentra is a subcutaneous version of infliximab.

Patients were more likely to stick with buprenorphine for 90 days, suggesting the value of virtual care. But researchers also found disparities in access.

Velsipity is the second oral S1P receptor modulator approved by the FDA to treat patients with moderate-to-severe ulcerative colitis. The wholesale acquisition cost is $6,164 for a 30-day supply.
The regulatory agency found deficiencies in its inspection of Alvotech’s Reykjavik, Iceland, facility. The company plans to resubmit its application once these are addressed.

The two-drug regimen of Braftovi and Mektovi was approved to treat patients who have a subset of non-small cell lung cancer, those with a BRAF V600E mutation.

The IV formulation has a wholesale acquisition cost of $2,115 per vial and will be available in the fourth quarter.
Alnylam Pharmaceuticals will no longer pursue this indication of Onpattro and will instead on focus on a label expansion for Amvuttra, which is in phase 3 development to treat patients with cardiomyopathy of ATTR amyloidosis.
The dosage of Zoryve for children 6 to 11 is the same as for those over the age of 12. The list price is same for both pediatrics and adults — $825 per tube.

Issues related to finding from an FDA inspection at a third-party filler have been resolved.
Galderma officials said they have already identified changes to its manufacturing process for relabotulinumtoxinA, which is being reviewed as a treatment for frown lines and crows feet.

In a confirmatory trial, Exkivity did not meet the primary endpoint in treating patients with non-small cell lung cancer with EGFR exon 20 mutations. It will remain available while Takeda works with the FDA on withdrawal timing.
FDA has set an action date of March 31, 2024, for marnetegragene autotemcel to treat infants with a serious and often fatal immunodeficiency.
Biogen is still evaluating launch timeline for Tofidence, and information on pricing will be available closer to launch.
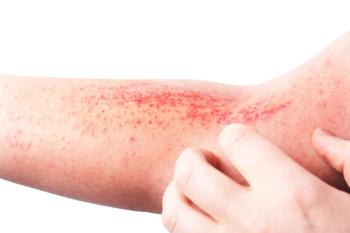
At issue are findings from the agency’s inspection of a third-party manufacturing company. The FDA indicated there were no concerns about the safety or labeling of lebrikizumab.

The two-component therapy of Pombiliti plus Opfolda to treat adults living with late-onset Pompe disease will have an annual list price of $650,000.
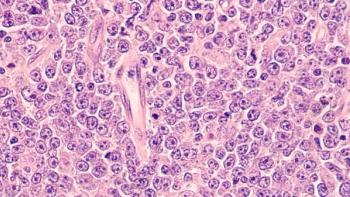
Odronextamab is a bispecific antibody to treat relapsed/refractory follicular lymphoma and diffuse large B-cell lymphoma. The target action date is March 31, 2024.

Exxua is approved to treat adults with major depressive disorder, but its labeling does not contain warnings about sexual function or weight gain.
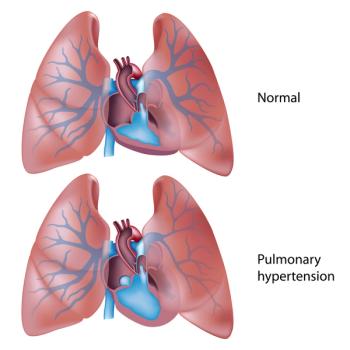
Sotatercept is a first-in-class therapy to treat the rare disease pulmonary arterial hypertension. The FDA has assigned a target action date of March 26, 2024.