
Daxxify enters the injectable botulinum toxin market as a twice-a year treatment for frown lines.

Daxxify enters the injectable botulinum toxin market as a twice-a year treatment for frown lines.

Dr. Reddy’s received a first-to-market 180 days of exclusivity for the 2.5 mg and 20 mg strengths of its generic lenalidomide capsules.

In a second committee meeting, FDA advisors supported approval of AMX0035 after the company presented additional analysis of phase 2 data of AMX0035 to treat patients with ALS. The Prescription Drug User Fee Act (PDUFA) target action date is Sept. 29, 2022.

A federal district court ruling today could jeopardize coverage of preventive services recommended by the U.S. Preventive Services Task Force. Some payers may be cautious about pulling back on full coverage of preventive services, but if the ruling stands, experts fear the undoing of a key provision of the Affordable Care Act will result in a patchwork of coverage — and a worsening of healthcare disparities.
If approved, SER-109 to treat recurrent C. diff infections could be the first-ever FDA approved oral microbiome therapeutic.

Fresenius Kabi expects to launch Stimufend as a prefilled syringe early in 2023.

The FDA would not issue an emergency use authorization for Eiger BioPharmaceuticals’ peginterferon lambda to treat patients with mild-to-moderate COVID-19.
If approved, NOV03 would be the first prescription eye drop to address excessive tear evaporation. The FDA has assigned a PDUFA action date of June 28, 2023.
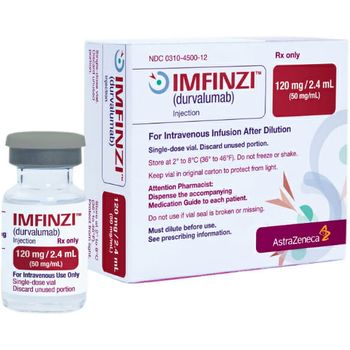
A phase 3 trial showed that Imfinzi in combination with chemotherapy reduced the risk of death by 20% compared with chemotherapy alone.

Konvomep, an oral liquid of omeprazole and sodium bicarbonate, will be available in the first quarter of 2023

With this approval, about 300 children between 12 months and 24 months will be eligible for treatment with Orkambi.
HHS is providing $11 million to Grand River Aseptic Manufacturing to accelerate manufacturing of the Jynneos monkeypox vaccine. The facility will be operational later this year.

Spevigo is a monoclonal antibody that inhibits interleukin-36 (IL-36) signaling approved to treat for adults with generalized pustular psoriasis flares.
This application is for a new formulation of carbidopa/levodopa extended-release capsules to treat patients with Parkinson’s disease.
Xenpozyme, the first disease-specific treatment for acid sphingomyelinase deficiency (ASMD), is expected to be available in the United States in the coming weeks at a wholesale acquisition cost of $7,142 per vial.

The approval of the updated bivalent boosters is based on data from clinical studies of the omicron variant BA.1, and animal data on the omicron BA.4 and BA.5 variant.

The Centers for Medicare and Medicaid Services released results today showing that on a per capita basis, the net savings for low-revenue accountable care organizations (ACOs) was higher than the net savings for high-revenue ACOs.

The target action date for the agency’s decision for efanesoctocog alfa, a factor VIII therapy, is Feb. 28, 2023.

Pemazyre is the first approved treatment specifically for patients with FGFR1 rearrangement, a rare blood cancer.
Imbruvica is the first approved treatment option for children under 12 with chronic graft versus host disease and the first BTKi therapy approved for pediatric patients.

Pending authorization, Moderna will be ready to ship the updated bivalent booster in September 2022.

The FDA has assigned a Prescription Drug User Fee Act action date of June 16, 2023.

Omnipod 5 is now available through retail pharmacies, as well as specialty and mail-order pharmacies.

The updated COVID-19 vaccine booster specifically addresses the omicron BA.4/BA.5 variants. If authorized, it will be available to ship immediately.

Novavax’s vaccine is the first protein-based COVID-19 vaccine authorized in the United States.