The new indication expands the patient population eligible for a Libtayo-based regimen to include combination treatment with chemotherapy irrespective of PD-L1 expression levels.

The new indication expands the patient population eligible for a Libtayo-based regimen to include combination treatment with chemotherapy irrespective of PD-L1 expression levels.

Cotellic, an oral inhibitor of MEK1 and MEK2, was approved to treat patients with histiocytic neoplasms based on a phase 2 trial conducted solely at Memorial Sloan Kettering Cancer Center.
This approval expands the use of Vemlidy to children 12 years of age and older. Vemlidy is already available for adults living with this chronic liver disease.
If approved, GSK’s vaccine could be the first available to help protect adults over the age of 60. The Prescription Drug User Fee Act date is May 3, 2023.

The withdrawal is related to the quality of the data collection and monitoring of the pivotal clinical trials by the clinical research organization.

The FDA cited COVID-19-related travel restrictions that have impacted the agency’s inspection of a manufacturing site in China.
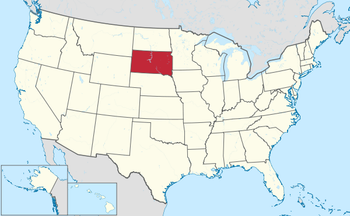
If South Dakota voters approve a ballot initiative on Nov. 8, that will leave 11 states that have not expanded their Medicaid programs under the ACA.

Epcoritamab is designed to simultaneously bind to CD3 on T cells and CD20 on B-cells

If approved, Perrigo’s Opill could be the first-ever nonprescription birth control pill.

Tecvayli targets BCMA and CD3 to activate the body’s immune system. It was approved for heavily pretreated patients with relapsed or refractory multiple myeloma.
If approved, SER-109 would be the first oral microbiome therapeutic available. The Prescription Drug User Fee Act action date is April 26, 2023.

Quizartinib, which is under review to treat adult patients with newly diagnosed acute myeloid leukemia who are FLT3-ITD positive, has a Prescription Drug User Fee Act date (PDUFA) of April 24, 2023.

Regulators have approved Imjudo, a monoclonal antibody that targets the activity CTLA-4, to be used in combination with Imfinzi, a PD-L1 inhibitor.

Novaliq’s CyclASol uses the EyeSol technology that allows for improved bioavailability and better efficacy. The PDUFA target action date set by the FDA is June 8, 2023.
The FDA has assigned a PDUFA goal date of June 16, 2023, for an expanded indication to reduce the need for septal reduction therapy (SRT), which is a procedure to treat hypertrophic cardiomyopathy.
Eylea is being reviewed as a treatment for retinopathy in premature infants.

Novavax’ protein-based vaccine is engineered from the genetic sequence of the first strain of SARS-CoV-2, the virus that causes COVID-19 disease.
If approved, tofersen will be the first treatment that targets a genetic cause of ALS. The updated PDUFA date is April 25, 2023.

If approved, valoctocogene roxaparvovec would be the first gene therapy in the U.S. for the treatment of severe hemophilia A. A PDUFA action date has been set for March 31, 2023.

The bivalent booster vaccines add the omicron variant BA.4 and BA.5 to the original SARS-CoV-2 along with a component of omicron BA.1.

The FDA has assigned a target action date of Feb. 11, 2023.

The submission is expected to be complete by the end of the year, and if approved, could be available in early 2024.

The FDA is asking for additional analysis related to the infusion device used with SPN-830, which contains apomorphine to treat patients with Parkinson’s disease.
Furoscix can be administered at home with the use of the On-Body Infusor, which delivers furosemide over five hours. It will be launched in the first quarter of 2023.
Regulators used a re-analysis of data from an observational case-control study of Tdap vaccine effectiveness to show that Boostrix given in the third-trimester prevented pertussis among infants.