If approved, Sandoz’ natalizumab would be the first biosimilar of Tysabri to treat patients with multiple sclerosis.

If approved, Sandoz’ natalizumab would be the first biosimilar of Tysabri to treat patients with multiple sclerosis.

If approved, tofersen will be the first treatment that targets a genetic cause of ALS. The FDA assigned a Prescription Drug User Fee Act action date of Jan. 25, 2023, but said it will hold an advisory committee meeting for this application.
If approved, Enhertu would offer a treatment option for patients with breast cancer who have a lower expression of HER2. The PDUFA action date is during the fourth quarter of the 2022.

Sandoz is seeking approval for a high-concentration formulation of Hyrimoz, which references AbbVie’s Humira.
The KEYNOTE-412 study did not meet its primary endpoint of event-free survival.
Pegcetacoplan is a targeted therapy to treat patients with age-related macular degeneration. The Prescription Drug User Fee Act (PDUFA) target action date is Nov. 26, 2022.

An independent data monitoring committee made the recommendation to stop both arms of the trial after a planned interim analysis found the trial didn’t meet its objectives.

If approved, this would be the first therapy to treat patients with Rett syndrome, a rare genetic neurological disorder mostly in girls.
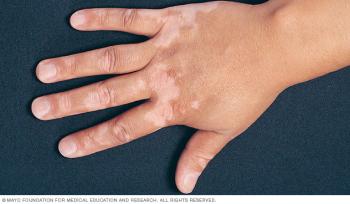
Opzelura is first therapy approved to treat patients with vitiligo, a disease that causes the loss of skin color.

Zonisade is the first oral liquid form of zonisamide to be approved by the FDA to treat patients with epilepsy.

Pfizer’s Xalkori is now approved for three indications, with the most recent being ALK-positive myofibroblastic tumors
The FDA has not been able to complete inspections of facilities because of COVID-19 restrictions.

The U.S. government pre-purchases 3.2 million initial doses of the Novavax COVID-19 vaccine.

Bludigo is the first indigotindisulfonate drug product approved by the FDA.

The FDA has assigned a Prescription Drug User Fee Act action date of May 12, 2023, for [vic-]trastuzumab duocarmazine (SYD985).
Piclidenoson is a first-in-class, A3 adenosine receptor agonist small molecule that inhibits the inflammatory cytokines interleukin 17 and 23.
The FDA approved a supplemental BLA for use of Krystexxa with methotrexate for the treatment of uncontrolled gout.
NOV03 (perfluorohexyloctane) is a first-in-class eye drop with a novel mechanism of action. If approved it will be the first to address signs and symptoms of dry eye disease.
Under the national vaccine strategy, the U.S. Department of Health and Human Services is expanding access to the monkeypox vaccine, Jynneos, in areas with the highest transmission and need.
If approved, mosunetuzumab could be the first T-cell engaging bispecific antibody for the treatment of any type of non-Hodgkin’s lymphoma. The FDA has assigned a PDUFA date of Dec. 29, 2022.
Lecanemab is monoclonal antibody that targets beta amyloid to treat mild cognitive impairment. The FDA has assigned a Prescription Drug User Fee Act (PDUFA) action date of Jan. 6, 2023.
The Peripheral and Central Nervous System Drugs Advisory Committee will meet for a second time in September 2022 to discuss new analysis of the data for AMX0035 for the treatment of patients with amyotrophic lateral sclerosis.

Qsymia is only available through a Risk Evaluation and Mitigation Strategy (REMS) program because of the increased risk for birth defects.

NRx Pharmaceuticals had been seeking emergency use for Zyesami for COVID-19 respiratory failure. The company will now focus on clinical research for its therapy for patients with bipolar disorder.

The submission seeks approval for the treatment of COVID-19 in both vaccinated and unvaccinated people at high risk for progression to severe illness from COVID-19.