
Fran Gregory, PharmD, MBA, VP of emerging therapies at Cardinal Health, talks about the importance of market access strategies in the cell and gene therapy space.

Fran Gregory, PharmD, MBA, VP of emerging therapies at Cardinal Health, talks about the importance of market access strategies in the cell and gene therapy space.

A future where healthcare may include augmented reality, robots and drones may be more fact than fiction, says George Van Antwerp, SVP of product innovation and strategic planning at Prime Therapeutics.

As healthcare AI evolves, there will be a greater emphasis on streamlining administrative tasks, drug production and service delivery, according to George Van Antwerp, SVP of product innovation and strategic planning at Prime Therapeutics.

Patients and providers found common ground on the importance of correct billing estimates but did not see eye to eye in terms of digital scheduling tool use, according to a new survey by Experian Health.

There is a lack of agreement on the clinical, psychological and social relevance of menopause between the transgender and gender diverse communities and medical professionals, according to the results of a study published this month in Menopause.

Women who undergo menopause before the age of 40 are more likely to experience cognitive decline, which may lead to dementia, according to new research.

Here’s what you missed this week on Managed Healthcare Executive.

Medicaid is the main healthcare coverage for people living with HIV in the United States, according to data compiled by KFF.

The difference between specialty drugs and traditional drugs and how each have vied for market competition, according to Jeff Casberg, MS, RPh, senior vice president of clinical pharmacy services at IPD Analytics.

In a Q&A with Jeff Casberg, MS, RPh, senior vice president of clinical pharmacy services at IPD Analytics, Casberg explains the evolution of specialty drugs, the future of GLP-1 competition and what to expect from the traditional drug pipeline.

Here’s what you missed this week on Managed Healthcare Executive.
Qfitlia (fitusiran) is the first therapy for both hemophilia A or B, with or without inhibitors, available in the United States.

Current observations and foresight into the world of biosimilars, GLP-1s, PBM and payer trends, according to Jeff Casberg, MS, RPh, senior vice president of clinical pharmacy services at IPD Analytics, and Doug Long, MBA, vice president of industry relations at IQVIA.

In United States veterans, antiretroviral resistance was associated with higher healthcare resource utilization, despite lower pharmacy-related costs, according to an abstract submitted to the Academy of Managed Care Pharmacy (AMCP) held last week in Houston.
The Xpert MTB/Ultra molecular diagnostic test may become a viable option for detecting tuberculosis (TB) in the stool of adults with HIV, according to the results of a recent study.

Here’s what you missed this week on Managed Healthcare Executive.

Originally developed to treat Type 2 diabetes, glucagon-like peptide 1 (GLP-1s) are now being studied to explore their effects on various other conditions, such as sleep apnea, substance use disorder and Alzheimer’s disease, according to panelists participating in a keynote session of the Academy of Managed Care Pharmacy annual meeting today in Houston.

Juan Carlos Scott, president and CEO of the Pharmaceutical Care Management Association (PCMA), and Marissa Schlaifer, RPh, M.S., vice president of policy at Optum.

Scott Biggs and Douglas M. Long of IQVIA delivered a keynote on 2024-2025 healthcare and pharmaceutical trends, highlighting drug shortages, pharmacy closures, and the United States dependence on imported pharmaceuticals.
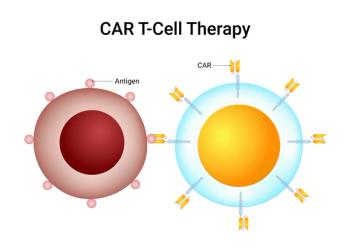
Balancing the cost, access to and risk management of CAR-T cell therapies is critical, according to a panel of experts who presented at the annual meeting of the Academy of Managed Care Pharmacy in Houston.

Digital solutions are needed in healthcare now so that more patients can get the access that they need, according to Vin Gupta, M.D., MPA, former chief medical officer of Amazon Pharmacy and a leading health policy expert.

Cardiac arrests in contemporary film are largely inaccurate when it comes to survival rate and etiology, according to a poster presented today at the American College of Cardiology conference held March 29 to 31 in Chicago.

The annual meeting of the Academy of Managed Care Pharmacy will take place in Houston from March 31 - April 3 at the George R. Brown Convention Center.

Here’s what you missed this week on Managed Healthcare Executive.

The Centers for Disease Control and Prevention’s Division of HIV Prevention are a critical source of funding and outreach for states, according to Terri L. Wilder, MSW, HIV/Aging Policy Advocate at SAGE, an organization that serves LGBTQ+ elders.

The fourth pair of denosumab biosimilars, Conexxence and Bomyntra, are expected to launch in the United States in mid 2025, as a result of a global settlement with Amgen, according to a company news release.

The Centers for Disease Control and Prevention (CDC) Division of HIV Prevention tracks outbreaks, provides education and establishes guidelines for the United States and the world, according to Terri L. Wilder, MSW, HIV/Aging Policy Advocate at SAGE, an organization that serves LGBTQ+ elders.
Blujepa is the first in a new class of oral antibiotics in 30 years. It is expected to launch in the second half of 2025 in the United States.
A Prescription Drug User Fee Act (PDUFA) target action date for apitegromab has been set for Sept. 22, 2025.

A growing mistrust of medical providers could be traced back to doubts about scientific validity and treatment costs, according to Luke Hansen, M.D., M.H.S., chief medical officer of Arcadia, a healthcare data analytics company.