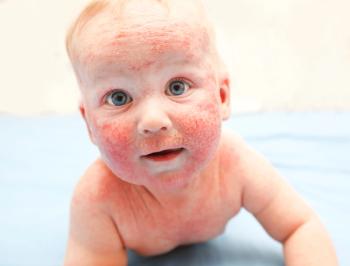
A South Korean study explored the link between lifestyle decisions beginning in infancy and persistent atopic dermatitis, citing exclusive breast milk diet and childhood obesity as potential factors.

A South Korean study explored the link between lifestyle decisions beginning in infancy and persistent atopic dermatitis, citing exclusive breast milk diet and childhood obesity as potential factors.
If approved, subcutaneous autoinjector Leqembi will be the only Alzheimer's medication that can be administered at home. The FDA has set a goal date of Aug. 31, 2025.

Centene Corporation’s Ambetter Health Solutions names Alan Silver as the first president of Individual Coverage Health Reimbursement Arrangements.
Here’s what you missed this week on Managed Healthcare Executive.

Blue Shield of California new CEO Lois Quam said she will focus on the challenges that members face with accessing healthcare.

Over 20 nonprofit veteran advocacy groups will receive $9 million over the next three years to address homelessness and mental health concerns in veterans.

A Guideline Development Group (GDG) meeting at the end of the month aims to establish new guidelines for lenacapavir, a twice yearly investigational pre-exposure prophylaxis (PrEP) injection.

Modern research shows women’s health issues go beyond reproductive health and highlight women’s elevated risk for autoimmune and cardiovascular disease – among other conditions.

Here’s what you missed this week on Managed Healthcare Executive.

Tafasitamab increased progression free survival by about 9 months when compared to placebo, according to recent study data.

Liraglutide injection is now approved for adult and pediatric patients ages 10 and up, proving another option amid the GLP-1 shortage.

Up to half of obstructive sleep apnea patients taking Zepbound had no symptoms after one year of treatment, averaging 25 fewer breathing interruptions per hour.

Here’s what you missed this week on Managed Healthcare Executive.

Although the primary endpoint was not met in a phase 2 study of women who used CBD before a scan, researchers say CBD does have some anti-anxiety effects.
If approved, Molbreevi could be the first FDA-approved treatment for patients with autoimmune pulmonary alveolar proteinosis, a rare lung disease.

Menopausal gay, lesbian and bisexual women are more likely to report recent sexual activity with less vaginal symptoms than heterosexual women of the same age, a new study suggests.
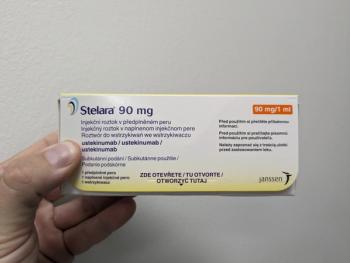
Originally approved in 2009, Stelara patents began expiring in 2023, leading to an influx of recent biosimilars.

If approved, clesrovimab would be the first and only FDA approved single dose immunization for infants approved in time for next year's RSV season, which lasts from October to April.
This latest approval is the second indication of the monoclonal antibody, Nemluvio. It was first approved in Aug. 2024 to treat patients with prurigo nodularis.

Here’s what you missed this week on Managed Healthcare Executive.
The new guidelines consist of 145 recommendations for treating and managing premature ovarian insufficiency syndrome, which affects an estimated 4% of women under the age of 40 worldwide.


Researchers have compiled a list of chemicals commonly found in plastics, including benzophenones, chlorinated paraffins and PFAS, known as “forever chemicals,” and they say there might be a connection to breast cancer.

Here’s what you missed this week on Managed Healthcare Executive.

Aerobic exercise, particularly static exercises like yoga and stretching, performed 70 to 90 minutes three times a week over eight to 10 weeks, can significantly improve sleep disorders in menopausal women.
The combination of Columvi, gemcitabine and oxaliplatin is the first CD20xCD3 bispecific antibody to show positive results in a randomized diffuse large B-cell lymphoma phase 3 trial. The FDA’s decision is expected by July 20, 2025.

The FDA has accepted a new drug application for aficamten, a new obstructive hypertrophic cardiomyopathy drug. A goal date has been set for Sept. 26, 2025.
Yesintek is the latest Stelara biosimilar to gain FDA approval and it will be available in February 2025.
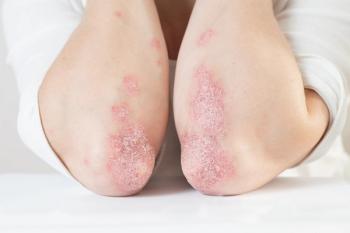
If granted, Tremfya will be approved to treat children ages six and under with severe plaque psoriasis and children ages five and under with juvenile psoriatic arthritis.

Here’s what you missed this week on Managed Healthcare Executive.