
COVID-19 care disruptions cut U.S. one-year cancer survival, adding 17,390 deaths; see which cancers and groups were hit hardest.

COVID-19 care disruptions cut U.S. one-year cancer survival, adding 17,390 deaths; see which cancers and groups were hit hardest.

Study finds transfusion access drives hospice choices in advanced blood cancers, urging new models that offer palliative transfusions with comfort care.

Recent research compares sleep aids in apps—sonic sleep sounds, white noise and pink noise—and warns weak evidence may waste money and delay proven insomnia care.

Patients using GLP-1 medications may notice facial changes due to fat loss. Vasyukevich stresses education and options for maintaining a youthful appearance.

Ten Medicaid tech companies pledge over $600 million in support to help states meet new community engagement requirements and modernize eligibility systems.

Explore the impact of ACA tax credits on enrollment, premiums and healthcare access as Congress debates subsidy extensions for 2026 and beyond.

Urgent care spending surges over 50% from 2018 to 2022, driven by increased visits, highlighting its vital role in healthcare access.

Proposed Medicare Advantage payment updates spark concern among insurers and investors, raising questions about risk adjustment and potential impacts on beneficiaries.

Lung cancer remains the leading cause of cancer deaths in the U.S. in 2026 and is expected to account for an estimated 229,410 new cases.


Medicare's 2024 initiative to address health-related social needs faces challenges, with over 25% of claims denied, highlighting barriers to effective care.

Physician groups prioritize patient care leadership and Medicaid reforms for 2026, addressing workforce challenges and public health issues in upcoming legislation.

Research reveals oral probiotics and synbiotics improve atopic dermatitis symptoms, while studies on other skin conditions remain limited.

In a conversation with Abby Kim, Pharm.D., of Prime Therapeutics, she shared that oncology pharmacists bridge critical gaps in precision medicine, enhancing patient care by streamlining biomarker testing and ensuring timely access to effective treatments.

New research revealed that many genetic variants linked to inherited retinal diseases rarely cause vision loss, challenging previous assumptions about their impact.

Combining NB-UVB light therapy with ruxolitinib cream enhances skin repigmentation in vitiligo patients, showing promising results without safety concerns.

CHOP partners with Soar Autism Center to enhance early autism care access, reducing wait times and providing integrated therapy services for families.

Discover the effectiveness of sonic sleep aids, revealing which audio tools truly enhance sleep quality and which may not deliver on their promises.
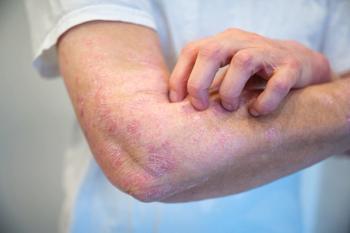
A recent study reveals ustekinumab outperforms adalimumab and secukinumab in drug survival for psoriasis, guiding treatment decisions for patients.

Independence Blue Cross announces three out of five 2026 Clinical Care Innovation Grants to enhance healthcare access and outcomes through innovative projects by leading health systems.

AI transforms how Americans navigate the complex healthcare system, offering timely support and enhancing decision-making for patients and providers alike.

Telehealth transforms mental health care for Medicare patients, replacing in-person visits while increasing overall spending, according to recent research findings.
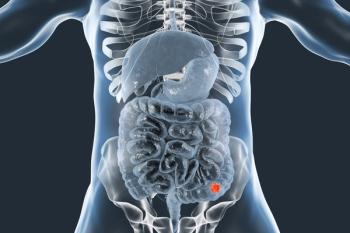
New research reveals how gastric cancer cells exploit WNT signaling for growth, revealing potential treatment targets to combat this deadly disease.
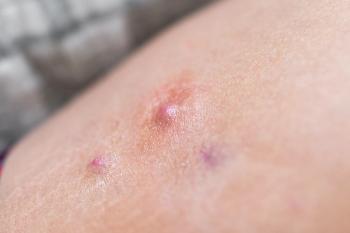
Discover groundbreaking treatments for prurigo nodularis, including vixarelimab and soficitinib, offering hope for effective symptom relief and improved quality of life.

Sunscreen remains essential for skin health, despite recent controversies. Experts emphasize proper use and broad-spectrum protection to prevent skin cancer.

Winter exacerbates atopic dermatitis in children, but effective management strategies and new treatments offer hope for improved skin health and comfort.
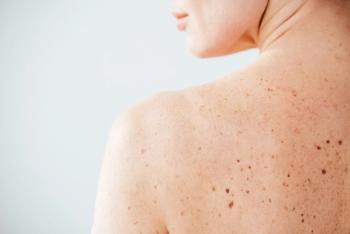
Discover groundbreaking advancements in dermatology from the 2025 Fall Clinical Conference, featuring innovative treatments for psoriasis, alopecia, and atopic dermatitis.

In 2025, atopic dermatitis care evolved with new treatments, prioritizing holistic methods and personalized strategies for improved patient outcomes.
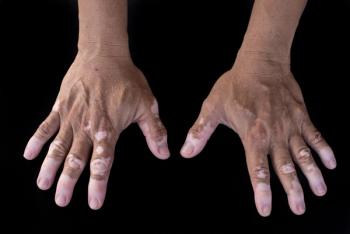
A study reveals that vitiligo significantly impacts quality of life and mental health in Japanese patients, highlighting the need for early psychological support.

Teledermatology revolutionized skin care in 2025, enhanced access and efficiency through virtual tools and AI while addressing safety and trust concerns.

Published: February 25th 2025 | Updated: February 28th 2025

Published: January 30th 2025 | Updated: January 31st 2025

Published: March 25th 2025 | Updated:

Published: November 4th 2020 | Updated:
Published: October 8th 2020 | Updated:

Published: May 2nd 2021 | Updated: