
Executive vice president of artificial intelligence, Altera Digital Health. Altera Digital Health provides digital services to healthcare clients.

Executive vice president of artificial intelligence, Altera Digital Health. Altera Digital Health provides digital services to healthcare clients.

The booking feature is now live for all Blue Shield employer-sponsored plans, Individual and Family Plans and Medicare members. The tool provides access to more than 1 million bookable appointment hours across a 90-day window.
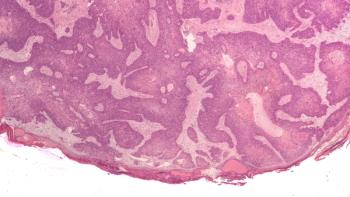
Cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer. While most cases are treatable, a small number can become serious and spread, leading to worse outcomes.

Manager of formulary operations and strategy, Yale Health. Yale Health is a not-for-profit, physician-led health maintenance organization that provides integrated, patient-centered care to the university community.

Researchers found that altering key enzymes involved in redox balance could disrupt the metabolism of Myc-driven lymphomas, which offers a potential new strategy to treat aggressive cancers.

Vice president and market executive at Highmark Health. Highmark Health is a national health and wellness organization serving nearly 50 million Americans.

CEO at Serve You Rx. Serve You Rx is a midmarket pharmacy benefit manager (PBM) for self-funded employers.

Bonnie Hui-Callahan, Pharm.D., says her career took off after not matching for a pharmacy residency—a setback that taught her resilience and led her to a better fit the second time around.

Senior director of clinical programs at Capital Rx Capital Rx is an enterprise health technology company and public benefit Capital Rx is an enterprise health technology company and public benefit corporationbthat provides various healthcare benefit administration solutions for commercial, Medicare and Medicaid plans.

In a new Wolters Kluwer report, it was revealed that 80% of respondents cited “optimizing workflows” as a top organizational goal, but only 63% felt prepared to use GenAI to achieve that.

Vice president of postacute care solutions at OneHome. OneHome is a postacute services business for Medicare Advantage members offering engaged, at-risk network oversight for home healthcare, skilled nursing facilities, durable medical equipment and infusion services.

Cassie Houff, MBA, says the most impactful change for U.S. healthcare would be “an overhaul to the fee-for-service payment system,” arguing that it doesn’t incentivize timely or necessary care.

Ernst & Young study shows 85% of participating organization leaders are seeing better patient outcomes, and nearly 75% report financial benefits when focusing on outcome-driven strategies.

Matthew Helbling says the turning point in his pharmacy career came when he took on a pilot program for medication therapy management—and discovered the power of connecting more deeply with patients.

Director of clinical effectiveness and operations at Prime Therapeutics LLC. Prime Therapeutics is a pharmacy benefits manager owned by 19 Blue Cross and Blue Shield plans.
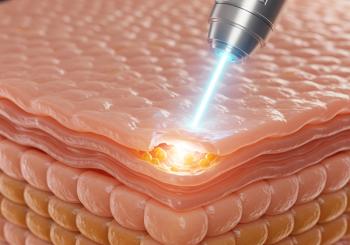
As researchers continue to explore more effective solutions, combining platelet-rich plasma (PRP) with fractional laser therapy has gained increasing attention.

Esteban Gallardo, Pharm.D., said his career path changed after early mentorship showed him how managed care pharmacists could make a broader impact on a larger scale.

Chief of pharmacy at CareAllies. CareAllies is a population health company that supports physician practices and health plans.

Kathryn Boger, Ph.D., said leaving academic medicine to co-found InStride Health was both terrifying and transformative. Boger reflected on advice she received early and said, “If you’re feeling scared, it’s a sign that you’re growing.” This wisdom continues to shape her path today.

Co-founder and chief clinical officer, InStride Health. InStride Health provides evidence-based care for children, teens and young adults with anxiety and obsessive-compulsive disorder (OCD) and their families.
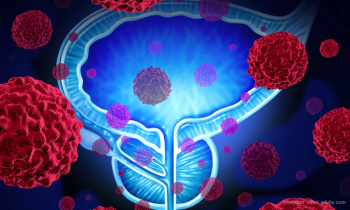
The approval is based on results from the phase 3 ARANOTE trial, which showed Nubeqa, when combined with androgen deprivation therapy (ADT), reduced the risk of radiographic progression or death by 46% compared to ADT with placebo.

Oren Mechanic, M.D., M.P.H., was one of Managed Healthcare Executive’s Emerging Leaders in Healthcare in 2022. We caught up with him as part of our “Where Are They Now?” series spotlighting the career paths of past emerging leaders.

In a recent conversation with Managed Healthcare Executive, Hunter shared reflections on his tenure, the challenges of expanding impact through partnerships and what he believes sets CareOregon apart from typical insurance organizations.

Alcon, a global eye care company known for products such as Systane and GenTeal Tears, plans to launch Tryptyr in the U.S. market in the third quarter of 2025. International rollouts are expected to follow.
A phase 2 trial found that adding bevacizumab to alectinib significantly delayed disease progression, protected against brain metastases, and improved quality of life in patients with advanced ALK-positive lung cancer.

In a conversation with Managed Healthcare Executive, Hunter expressed that work requirements are an effective way to manage Medicaid eligibility, but what is proposed will cause more harm to those who can't afford commercial healthcare.

In this second part of a video series, Managed Healthcare Executive caught up with CareOregon CEO Eric C. Hunter, who voiced concern about the consequences of rising anti-DEI views and potential federal funding cuts.

Led by the University of Queensland’s research facility, Frazer Institute, the trial examining the drug ASITI-201 is the first of its kind in the world and has already dosed its first five participants.

Developed by Sarepta Therapeutics, Elevidys is a one-time, intravenous treatment that uses adeno-associated virus (AAV) technology to deliver a gene designed to produce a shortened form of the dystrophin protein, known as Elevidys micro-dystrophin, directly to skeletal muscle.