
The Inflation Reduction Act’s limit on Medicare Part D spending can lead to savings for patients prescribed oral chemotherapy drugs.

The Inflation Reduction Act’s limit on Medicare Part D spending can lead to savings for patients prescribed oral chemotherapy drugs.

This analysis finds that 57.4 million adults under the age of 65 could potentially be eligible for GLP-1 drugs based on currently approved FDA indications, including 36.2 million people with obesity.

The private-label biosimilar will be available at an 80% discount off Stelara. Evernorth has not released information about which company will be producing the biosimilar.

Outcomes-based agreements can take away clinical uncertainties, so payers have a guarantee that they're not spending money on something that’s not going to work.
The growth of out-of-pocket and insurer/negotiated prices paralleled one another until 2016 when negotiated and insurer prices began declining

Sebetralstat would be the first oral, on-demand treatment for hereditary angioedema if approved.

The new program provides large employers with access to Capital Rx’s technology platform for claim adjudication, invoicing, and reimbursement.

MedImpact is not requiring patients currently taking Humira to switch, and all three products are on a preferred tier.
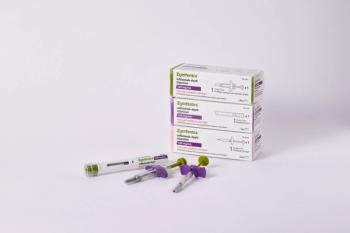
Zymfentra is a subcutaneous infliximab product used to treat ulcerative colitis and Crohn’s disease. It has a price of $6,181.08 for two shots over four weeks.

The FDA has given mirdametinib a Prescription Drug User Fee Act (PDUFA) action date of Feb. 28, 2025, to treat patients with neurofibromatosis type 1 with tumors that grow along the peripheral nerve.

Through the PfizerForAll platform, patients can connect with a healthcare professional, find and book vaccines, order tests, and find medication savings information.
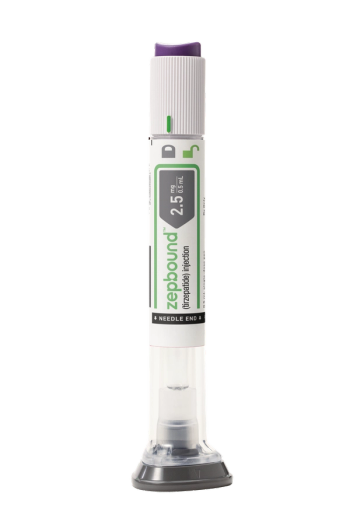
The monthly price of the 2.5 mg Zepbound single-dose vial is $399 and the 5 mg dose is $549, which Lilly officials said is in line with the savings program for without insurance.

Beginning in 2025, Express Scripts will favor biosimilars of Humira, including its own private-label version.
The updated vaccines include the KP.2 strain of the Omicron variant, which is believed to be contributing to the increases in COVID-19 infections this summer.

Avalere’s Kylie Stengel talks about the regional shifts in formularies and utilization management in Medicare Part D prescription drug plans.

The FDA has assigned a Prescription Drug User Fee Act of Dec. 20, 2024, for Zynquista (sotagliflozin) to control glycemic levels in adults with type 1 diabetes and chronic kidney disease.
The FDA identified at a third party fill and finish manufacturer. The issues have been resolved, and reinspection is expected in the next few months.

This therapy is a first-in-class treatment for chronic graft-versus-host disease, which develops in roughly 42% of those who receive a stem cell of bone marrow transplant.
Livdelz is an oral therapy to treat patients with primary biliary cholangitis, a progressive autoimmune disease. It is expected to be available in pharmacies next week.

This first-in-class treatment targets multiple disease mechanisms such as itch and inflammation associated with this condition.

Prademagene zamikeracel is a cell therapy in development to treat patients with recessive dystrophic epidermolysis bullosa (RDEB), a rare connective tissue disorder.

Zymfentra launched in March 2024 as the first subcutaneous formulation of infliximab for patients with ulcerative colitis and Crohn’s disease. It has a list price of $6,181.08 for two shots over four weeks.

No information, however, is available about which insurance plans are providing coverage of Anktiva or what the utilization management requirements are.
Enzeevu is biosimilar to Eylea (aflibercept), which was FDA-approved in 2019.

In June, an FDA advisory committee voted against approval of midomafetamine capsules (MDMA) for adults living with posttraumatic stress disorder (PTSD).

Neffy is the first epinephrine product that does not need to be administered by injection and the first new epinephrine delivery method in 35 years. It will be available at a starting price of $25.