
Regulators say, however, the benefits of vaccination with Abrysvo and Arexvy in preventing respiratory syncytial virus continue to outweigh their risks.

Regulators say, however, the benefits of vaccination with Abrysvo and Arexvy in preventing respiratory syncytial virus continue to outweigh their risks.

Effective Jan. 1, 2025, Independence Blue Cross has begun excluding coverage for 18 months for therapies — but not cancer drugs — that have an accelerated approval based on a surrogate endpoint.

Deramiocel is a cell therapy that has healing effects in muscle cells. If approved, deramiocel would be a once-quarterly therapy and the first specifically approved for cardiomyopathy in patients with Duchenne muscular dystrophy.
FDA officials have set a review date of June 30, 2025, to review avutometinib in combination with defactinib to treat patients with recurrent low-grade serous ovarian cancer.

In November 2024, a regulatory advisory board said the benefits of Zynquista does not outweigh the risks in adults with type 1 diabetes and chronic kidney disease.

Opdivo Qvantig is the first subcutaneously administered PD-1 inhibitor approved by the FDA. It is expected to be available in early January 2025 and will be priced at parity to the IV version of Opdivo.

Liraglutide injection is now approved for adult and pediatric patients ages 10 and up, proving another option amid the GLP-1 shortage.

Up to half of obstructive sleep apnea patients taking Zepbound had no symptoms after one year of treatment, averaging 25 fewer breathing interruptions per hour.
If approved, Molbreevi could be the first FDA-approved treatment for patients with autoimmune pulmonary alveolar proteinosis, a rare lung disease.
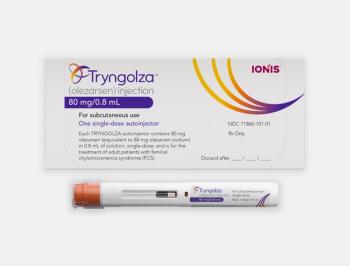
Tryngolza is once-monthly, subcutaneous RNA-targeted therapy and is expected to be available by the end of the year with a price of $595,000.

The Institute for Clinical and Economic Review assessed the formularies of 11 payers, covering 57 million people, to determine access for drugs that the organization had reviewed in 2022 for cost-effectiveness.
Originally approved in 2009, Stelara patents began expiring in 2023, leading to an influx of recent biosimilars.
The boxed warning follows a safety warning the FDA issued in September about the risk of liver injury from the use of Veozah to treat hot flashes.

A new bill in Congress would require pass-through prices in both Medicare, and Medicaid and require transparency of PBM practices in the commercial space.

If approved, clesrovimab would be the first and only FDA approved single dose immunization for infants approved in time for next year's RSV season, which lasts from October to April.
This latest approval is the second indication of the monoclonal antibody, Nemluvio. It was first approved in Aug. 2024 to treat patients with prurigo nodularis.
In 2023, there was lower net spending and prices on adalimumab, which was likely because of rebates from AbbVie, Humira’s manufacturer, a new analysis finds.

The Institute for Clinical and Economic Review has identified five drugs — Biktarvy, Darzalex, Entresto, Cabometyx, and Xeljanz — with prices increases that are not supported by new clinical evidence, with a total of $815 million in added costs to U.S. payers in 2023.

Beginning in January 2025, Optum Rx will remove from several Humira biosimilars from its formulary, including Hyrimoz (adalimumab-adaz), the unbranded Hyrimoz and Cyltezo (adalimumab-adbm).
The biggest advantage of suzetrigine would be that it helps patients avoid the use of opioids for acute pain. The FDA’s goal date for suzetrigine is Jan. 30, 2025.
The combination of Columvi, gemcitabine and oxaliplatin is the first CD20xCD3 bispecific antibody to show positive results in a randomized diffuse large B-cell lymphoma phase 3 trial. The FDA’s decision is expected by July 20, 2025.

The FDA has accepted a new drug application for aficamten, a new obstructive hypertrophic cardiomyopathy drug. A goal date has been set for Sept. 26, 2025.
Yesintek is the latest Stelara biosimilar to gain FDA approval and it will be available in February 2025.
If granted, Tremfya will be approved to treat children ages six and under with severe plaque psoriasis and children ages five and under with juvenile psoriatic arthritis.

Vutrisiran is the generic form of previously approved Amvuttra. The FDA’s target date for the treatment of transthyretin amyloidosis with cardiomyopathy is March 23, 2025.

Whether this proposed rule actually has any impact remains to be seen, especially with a new administration led by Donald Trump’s new health appointees.