The FDA is reviewing two novel therapies: a psychedelic-assisted therapy for PTSD with a target action date of Aug. 11, 2024, and therapy for schizophrenia that does not directly block dopamine receptors with an action date of Sept. 26, 2024.

The FDA is reviewing two novel therapies: a psychedelic-assisted therapy for PTSD with a target action date of Aug. 11, 2024, and therapy for schizophrenia that does not directly block dopamine receptors with an action date of Sept. 26, 2024.

Ventegra, which provides drug benefit solutions, is also converting to a nonprofit status, which will be completed June 30, 2024.
The FDA is currently reviewing tarlatamab for small-cell lung cancer with a review date in June and datopotamab deruxtecan for non-small cell lung cancer with a review date in the fourth quarter.

FDA officials are looking for additional information about the infusion device used to deliver apomorphine.

Caps on Medicare Part D cost sharing as a result of the Inflation Reduction Act, could reduce members’ financial incentive for switching to a biosimilar, suggests the newest Samsung Bioepis Quarterly Biosimilar Market Report.

The quality-adjusted life-years tool has been misunderstood, Dr. Joshua Cohen says. QALYs are a measure of the value that comes from restoring a person to health, not about discrimination.

Approved in late September 2022, Relyvrio will no longer be available for new amyotrophic lateral sclerosis (ALS) patients.

The FDA is also considering a separate BLA for a 2 mL syringe. Bimzelx is already available to treat plaque psoriasis as a 1 ml syringe; a dose is two subcutaneous injections with a cost of $7,200 per syringe.
Zevtera was approved to treat Staphylococcus aureus bloodstream infections, acute bacterial skin and skin structure infections; and community-acquired bacterial pneumonia.


Fanapt is now approved to treat adults acute manic or mixed episodes associated with bipolar I disorder. It is also available to treat schizophrenia.
Bacillus cereus contamination in Atovaquone Oral Suspension, which treats AIDS-related pneumonia, was found during stability testing at a third-party lab.

In 2025, the out-of-pocket cap and other Part D related provisions in the Inflation Reduction Act are projected to save women in Medicare an average of 28% in out-of-pocket costs, according to a new analysis from the Office of the Assistant Secretary for Planning and Evaluation.
Voydeya was approved for patients with paroxysmal nocturnal hemoglobinuria rare, progressive blood disorder that results in the developed of abnormal blood cells that are missing important proteins.

The recommended maximum daily dose of Vancomycin is up to 2 grams a day. The overfilled vials could result in patients receiving up to 4 grams a day.

Foluso Agboola discusses the framework that the Institute for Clinical and Economic Review has developed for assessing whether clinical trial populations represent the real-world patient population.

Vemlidy is now approved to treat patients 6 years and older with chronic hepatitis B virus infection.
Vafseo, an oral therapy to treat patients with anemia due to chronic kidney, will be available in January 2025. About 40% of patients who could be treated with Vafseo have coverage through Medicare Advantage plans.

Ozempic and Rybelsus could become a target for Medicare price negotiation because of high spending and additional approved uses of the GLP-1 therapies.
Vivjoa is an azole antifungal to reduce the incidence of chronic vaginal yeast infection in postmenopausal women.
Sotatercept — now with the brand name Winrevair — will be available through specialty pharmacies by the end of April. The price of Winrevair is $14,000 per vial.

Medicare Part B beneficiaries may save between $1 and $3,575 per average dose for the 41 drugs whose prices have risen higher than inflation.
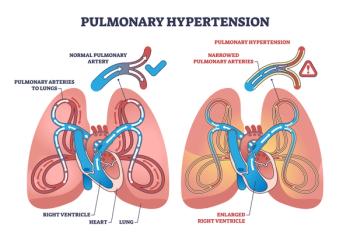
J&J’s Opsynvi is single-tablet combination of macitentan, an endothelin receptor antagonist, and tadalafil, a PDE5 inhibitor. It will be priced on parity with Opsumit, which is also a J&J product to treat patients with pulmonary arterial hypertension.
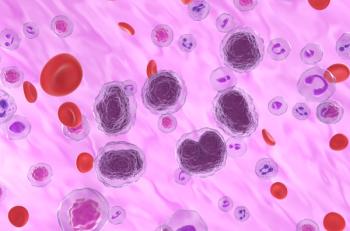
FDA officials indicated that the confirmatory trial for odronextamab for patients with follicular lymphoma and diffuse large B-cell lymphoma should be under way before resubmission.
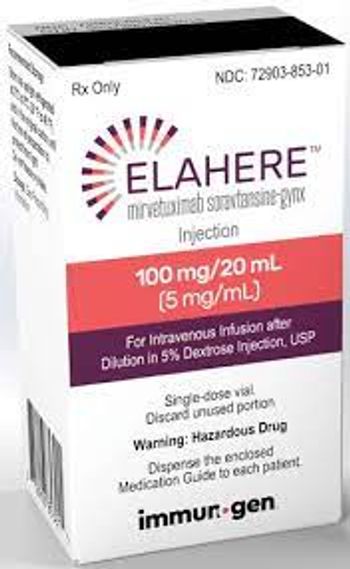
The full approval was based on the confirmatory phase 3 MIRASOL trial, which showed that Elahere resulted in a 33% reduction in risk of death and a 35% reduction in the risk of cancer progression.

Medicare and Medicaid will cover Wegovy for its recently approved indication to reduce the risk of cardiovascular death, heart attack and stroke in adults with cardiovascular disease and obesity.