For Magellan’s clients, Ozempic had the highest trend of any product in 2022, and Wegovy was the fifth highest drug in terms of trend. Diabetes as a whole is expected to become the top condition leading pharmacy trend by 2024.

For Magellan’s clients, Ozempic had the highest trend of any product in 2022, and Wegovy was the fifth highest drug in terms of trend. Diabetes as a whole is expected to become the top condition leading pharmacy trend by 2024.
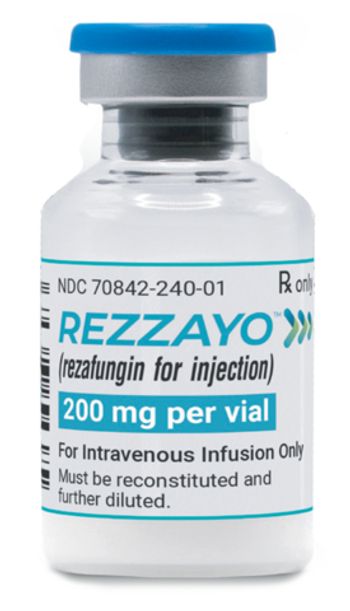
Rezzayo is a novel once-weekly antifungal to treat invasive candidiasis, a serious infection that can affect the blood, heart and brain. It will have a wholesale acquisition cost of $1,950 per vial.

MacKay Jimeson of the advocacy organization Patients Rising talks about CMS’ decision to limit coverage for Alzheimer’s drugs to those with full approval.

Accountable care organizations (ACOs) use digital health to complement traditional resources.

Renee Rayburg talks about the specialty drug spend, which grew 14.1% in 2022. PSG analysts predict increases will continue to be in the low double-digits for the next three years.

Sara Izadi, Pharm.D., of Capital Rx, talks about Rx Reverse, an integrated clinical program designed to help members reverse diabetes and fight obesity.

The FTC says that prior statements and studies about the PBM industry no longer reflect current market realities.

An analysis by Biosimilars Council has found that the reference product Lantus accounts for 54% of new prescriptions and is 78% of total market volume but demand for the unbranded interchangeable insulin glargine is increasing.

The newly formed Peterson Health Technology Institute was launched with $50 million to analyze clinical benefits and economic impact of new health technologies.
About 36% of Medicare Part D enrollees will benefit from the Inflation Reduction Act’s out-of-pocket caps for prescription drugs, which will go into effect in 2025.

Most private health insurance plans are required to cover vaccines for COVID-19 without patient cost-sharing.
In the short term, two gene therapies now under review at the FDA — lovo-cel and exa-cel — can reduce the frequency of painful crises in patients with severe sickle cell disease.
KFF analysis has found that 10 drugs account for 22% of all Medicare Part D spending in 2021.

Prime Therapeutics’ analysis of claims data suggests patients require support to ensure continued use and lifestyle modification.

A 30-day supply of Brenzavvy is available from Cost Plus Drugs for $47.85.

Beginning in January 2024, if GoodRx shows a lower CVS price than a member’s plan price, that lower price will automatically be given.

About half of Navitus’ commercial clients saw a drug spend decrease compared with 2021.

Governor Phil Murphy has signed three pieces of legislation that would require PBMs to use prescription drug rebates to lower premiums and out-of-pocket costs for consumers and prevent the practice of spread pricing.

Express Scripts will also add Sandoz’s unbranded version of adalimumab to its National Preferred Formulary. The Humira biosimilar Amjevita was added previously.
Medicare will cover Alzheimer’s drugs with full approval, including Leqembi — if the physician and patient participate in a real-world registry trial to gather additional data.
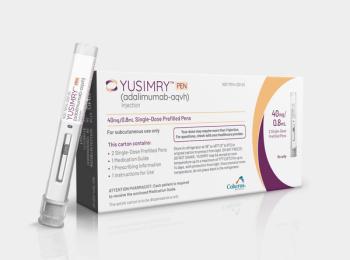
RxPreferred will offer Yusimry, a biosimilar of Humira, at a price of $569.27 plus dispensing and shipping fees. This price represents a 92% discount off Humira’s monthly price.
Most of the Humira biosimilars are available with two pricing options. Of the larger PBMs, only Optum Rx so far has weighed in on a coverage decision.

Similar to Amgen’s Amjevita, Hulio will be offered at a list price of 5% below and 85% below the current Humira list price.
The increase is expected to be somewhat offset by the impact of biosimilars coming to market and more care being provided in outpatient settings.

How the insurance industry denies claims; consumers confused by complexity and red tape; and how the ALS drug Relyvrio came to be.