
Zurzuvae, the first oral therapy for postpartum depression, will launch in December.

Zurzuvae, the first oral therapy for postpartum depression, will launch in December.

Using an AI platform, Magellan Health was able to better support providers in prescribing behavioral health medications and addressing medication problems, which reduced pharmacy costs and increased adherence.

First in class, biosimilars, oncology drugs and even some generics have been added to Optum Rx’s list of exclusions for 2024.

The Institute for Clinical and Economic Review’s fair access report found that for the drugs assessed, formularies provided fair access, but it was difficult to determine how well that translates into real-world access and affordability for patients.
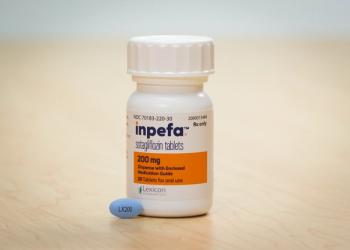
Express Scripts has also included the heart failure drug Inpefa on its Medicare formularies.

A poster from AMCP Nexus quantified tends in biosimilar coverage within Tufts Medical Center, finding that payer coverage of these products has increased but individual preferences among payers still varies.
The Humira biosimilar Yuflyma will be available through CarePartners specialty pharmacy business, which has 10 million plan members.

The mean lag time between FDA approval and insurance coverage for 89 cancer drugs ranged from 2.3 months to 8.2 months. But cancer drugs approved more recently were more likely to have shorter coverage lags.

The increase is close to inflation and wage growth but much steeper than the atypically small increase in 2022.

Researchers in this review suggest clinical trials of biosimilars include switching studies to assess the pharmacokinetics of the biosimilar candidate.

Whether Opill (norgestrel) truly broadens access to daily contraception will depend on Medicaid coverage, requirements under the ACA and pharmacist prescribing.

Yuflyma is on Ventegra’s public and private formulary on parity with the reference product Humira.

Insurers and PBMs now have to follow a 2020 rule in which accumulator programs can only be applied to brand name drugs that have generic equivalents available.
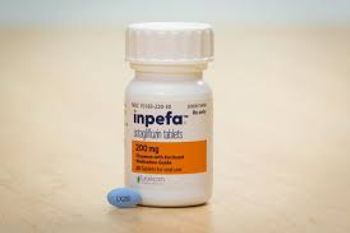
Inpefa is an inhibitor of both SGLT2 and SGLT1, approved to reduce the risk of cardiovascular death and hospitalization for heart failure in adults. The wholesale acquisition cost is $598 per month.
Patients who continued with their rheumatoid arthritis biologic medication tended to spend less on healthcare, had a lower chance of being hospitalized, and had shorter hospital stays.

Mark Campbell, Pharm.D., RxBenefits’ senior vice president of clinical solutions, talks about how Medicare’s drug price negotiations could have a ripple effect on pharmaceutical company innovation and prices on the commercial side.

ICER has weighed in on two of the 10 drugs selected for negotiations: Eliquis and Xarelto, which are among Medicare Part D’s highest spending drugs.

Once Medicare’s negotiated price for prescription drugs goes into effect, payers have more of an incentive to steer patients to lower-cost alternatives.

Aetna, Cigna, Humana, United Healthcare — some of the largest providers of Medicare Advantage plans — have released updates to prescription drug programs.
The recent study also found that Jardiance for patients with heart failure with preserved ejection fraction could be cost-effective if discounted by 29%.

The class action lawsuit against CVS Health, Caremark, and Aetna was announced earlier today.
Iyuzeh can be stored at room temperature. It has a list price of $299 for a month’s supply.

ICER adds a more formal process to evaluate the diversity of clinical trials and an assessment of a product’s impact on patient and caregiver productivity. ICER also plans to evaluate how newer methods — which would consider the change of a drug’s price over time and disease severity — can be applied to its value assessment.

Non-White patients who used copay cards were significantly more likely to encounter copay adjustment programs, such copay accumulators and maximizers, compared with their White counterparts.
Laurie Sobel, associate director for women’s health policy at KFF, moderated a panel discussion about the challenges associated with providing insurance coverage for the first FDA-approved oral contraceptive, Opill.