
Experts say ordinary sunglasses aren't protective enough for viewers of the solar eclipse on Monday. You need those certified eclipse glasses. Ten-packs were selling on Amazon today for $12.99.

Experts say ordinary sunglasses aren't protective enough for viewers of the solar eclipse on Monday. You need those certified eclipse glasses. Ten-packs were selling on Amazon today for $12.99.

In 2025, the out-of-pocket cap and other Part D related provisions in the Inflation Reduction Act are projected to save women in Medicare an average of 28% in out-of-pocket costs, according to a new analysis from the Office of the Assistant Secretary for Planning and Evaluation.

We need your views! Help us help you.

A recent survey on primary care spending showed the U.S. lagging behind.

This week, the FDA approved two therapies for pulmonary arterial hypertension: Merck’s first in class therapy Winrevair and J&J’s oral combination tablet Opsynvi. The agency also approved Vafseo, a new treatment for anemia in kidney disease. The FDA granted full approval to the cancer drug Elahere and extended the indication of Vemlidy to children. Regulators also issued a CRL for odronextamab for lymphoma indications

People with HIV who smoke have a life expectancy 12.3 years shorter than non-smokers.

The results of this week’s poll about the Affordable Care Act.

In this second part of a two-part video series, MHE editors spoke with Muthiah “Muthu” Vaduganathan MD, MPH, consultant cardiologist at Brigham and Women's Hospital and a member of faculty at Harvard Medical School, about the cost effectiveness of SGLT2 inhibitors.

Ramin Bastani, founder and CEO of Healthvana, based in Los Angeles, spoke about the current state of HIV in the United States and how his company is helping lower infection rates through patient engagement.

The Departments of Health and Human Services (HHS), Labor, and the Treasury, released final rules on short-term, limited-duration insurance (STLDI) and independent, non-coordinated excepted benefits coverage, addressing issues related to the low-quality insurance, or "junk insurance" plans.

Editors of Managed Healthcare Executive spoke with Muthiah “Muthu” Vaduganathan MD, MPH, consultant cardiologist at Brigham and Women's Hospital and a member of faculty at Harvard Medical School, about the evolving treatment landscape for heart failure, emphasizing the use of SGLT2 inhibitors alongside other foundational therapies.

With aging populations, osteoporosis rates are set to increase, and researchers are looking for the next innovative drug to help prevent or manage the bone disease as there is currently no drug that completely does so effectively, according to a recent study published in Advanced Science.

Medicare Part B beneficiaries may save between $1 and $3,575 per average dose for the 41 drugs whose prices have risen higher than inflation.

New development comes after the FDA’s Mar. 8 Wegovy approval for patients at risk for heart attack and stroke.

In a poster presented at the 2024 American Pharmacists Association Annual Meeting and Exposition, researchers found that state-level PBM reform focused on more transparent drug pricing, better patient access to prescription drugs, and more stringent auditing and reporting requirements.
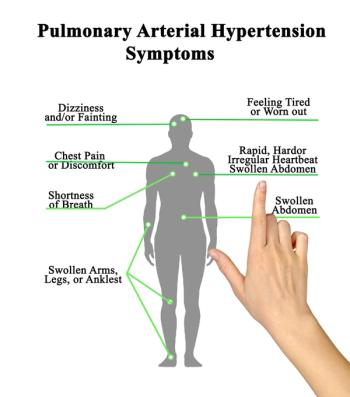
J&J’s Opsynvi is single-tablet combination of macitentan, an endothelin receptor antagonist, and tadalafil, a PDE5 inhibitor. It will be priced on parity with Opsumit, which is also a J&J product to treat patients with PAH.

The 14th anniversary of the Affordable Care Act was this past Saturday.

New approvals this week include a $4.25 million gene therapy, a drug that treats hypertension in a new way, a nonsteroidal drug for Duchenne, and an oral drug for aggressive leukemia. The agency has set an action date for resubmitted Lymphir in rare skin cancer, and Celltrion has launched Zymfentra, a subcutaneous form of infliximab.

The health industry is changing rapidly, and organizations that don’t adapt are going the way of the Model T. By offering new solutions and capabilities, successful organizations are supporting their customers with whole-person, full-journey care.

Given the role of patient self-management in diabetes care, there has been significant investment in digital diabetes management solutions.

The results of this week’s poll about whether copay accumulators should be banned.

High-deductible health plans are supposed to make patients smarter shoppers for healthcare services. But result reported in JAMA Network Open today add to the evidence that people with chronic conditions such as diabetes may put off care when deductibles are high, increasing the risk of complications and worsening of the disease.

Although there is a suspected connection between malaria and HIV, no malaria control strategies have been developed specifically for people living with HIV.

Results from the 2024 State of Patient Access report by the PAN Foundation shared that patients with chronic health conditions, in particular, face barriers to accessing quality care due to costs, coverage gaps and social challenges with healthcare professionals (HCPs).

Researchers found that Black and Hispanic dementia patients had the highest Medicare expenditures.

Lenmeldy is the first approved gene therapy to treat children with juvenile metachromatic leukodystrophy, a life-threatening inherited disease of the body’s metabolic system.

About 20 states have moved to ban copay accumulators.

In this first part of a two-part podcast episode, Managed Healthcare Executive's Managing Editor Peter Wehrwein kicks off our new podcast series "DC Roundtable," with guests Margaret "Meg" Murray, CEO of the Association of Community Affiliated Plans, and member of MHE's Editorial Advisory Board, and Ceci Connolly, president and CEO of the Alliance of Community Health Plans, for a discussion on healthcare policy issues.

The average sickle cell disease patient on Medicare paid $22,600 for care in 2021.

The FDA this week approved a few firsts: the first treatment for NASH and the first CAR-T cell therapy for CLL/SLL. Other approvals include Tevimbra for esophageal cancer; Livmarli for second liver disease indication; and Praluent for children with genetic form of high cholesterol. The FDA also issued a CRL for monthly MS drug. Additionally, Celltrion submitted an application for an interchangeable Xolair biosimilar.