
Cerdá, a member of the Managed Healthcare Executive® editorial advisory board, is part of a new wave of leadership at the Philadelphia-based insurer led by CEO George Deavens.

Cerdá, a member of the Managed Healthcare Executive® editorial advisory board, is part of a new wave of leadership at the Philadelphia-based insurer led by CEO George Deavens.
United States healthcare spending rose 9.7% in 2020, while medication spending is increasing by more than 18%.

Healthcare professionals need to make patients the primary decision-makers in their own care and actively support their efforts to achieve their care goals.
Most (54%) respondents indicated that they believe the FDA made a mistake when it approved the controversial Alzheimer’s drug in June 2021.
The limit is four tests per household.

Telehealth, efforts to address social determinants of health and a focus on health disparities are likely to be among the top priorities in population health this year.


The FDA okayed two Janus kinase 1 (JAK1) JAK inhibitors January 14 the for atopic dermatitis: Cibinqo and Rinvoq.

Forty percent of the respondents to our annual State of the Industry survey expect vertical integration of providers to increase this year.
Walmart seems to be pulling back and opioid litigation looms.

The use of digital devices has surged significantly as people spend more time at home, with Americans logging an average of 13 hours per day watching screens. That compares to between seven and 10 hours per day before the COVID-19 pandemic started, with the increase in screen time likely contributing to more exposure to blue light.

In a research letter published in JAMA Health Forum, researchers say most of the cost figures for Aduhelm have left out the additional MRI scans and other services that will be required because the drug is associated with the development of amyloid-related imaging abnormalities (ARIAs).

Organizations setting up hospital-at-home programs need to be aware of the associated risks and mitigation best practices.
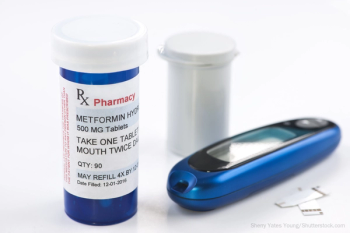
Problems with N-nitrosodimethylamine (NDMA)-contaminated metformin were identified by the FDA in 2020. Viona Pharmaceuticals announced in earlier this month that it was recalling 23 lots of the popular diabetes medication.

The biggest concern of the respondents to our State of the Industry survey was misinformation about vaccines on social media.

The company is expanding the reach of Amazon Care and partnering with health systems and senior living companies to support at-home care.

For many people, telehealth is an inviting option. But some insurers are offering health plans that make it the main way by which people receive primary care.

Cost pressures may increase as the Medicare Hospital Insurance Trust Fund runs low, but the reliance on telehealth that the pandemic wrought is likely to continue, especially for mental health services.

Briana Contreras, editor of Managed healthcare Executive, spoke with Dr. Ian Tong, chief medical officer of Included Health in today’s episode. The two discussed telehealth use vs. in person care, and more specifically, the racial and ethnic differences in this use of care. They concluded the conversation with how telehealth use has continued to expose more benefits for all patients and for the industry through examples like improvements in reimbursement policies and in addressing health equity issues.

A large majority of respondents see a future of endemic COVID-19, but the survey also identified some confidence that the current crop of vaccines will protect against new variants.
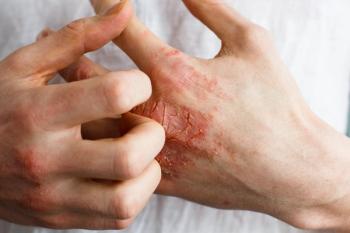
Leo Pharma's Adbry (tralokinumab-ldrm) is the second, FDA-approved biologic for atopic dermatitis. Dupixent (dupilumab) was the first.

CMS has proposed covering the anti-amyloid treatment only if the person taking it is enrolled in a clinical trial.

Results of our survey show some tempering of expectation for addressing healthcare disparities and what can be accomplished this year.

But the future of value-based care in oncology is uncertain with no certain successor to the Oncology Care Model in sight.

Remote care technology and drug price limits garnered the most votes for having the biggest effect on the cost and delivery of U.S. healthcare.
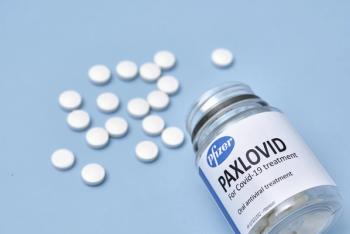
Smaller doses are recommended for people with moderate renal impairment and the treatment should not be taken by people with serious kidney disease. There are also drug interactions to be aware of because Paxlovid is an inhibitor of CYP3A.
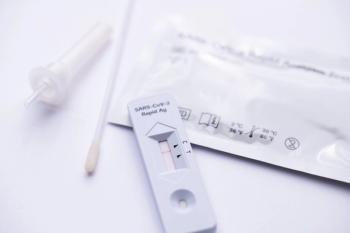
As the need for rapid tests increase, the nation's largest non-profit patient safety organization evaluated seven COVID-19 rapid tests and found serious gaps in their usability. Results shared that none of the tests were rated as excellent and some had noteworthy usability concerns.
Most of the respondents to our question about optimism of notable improvements in healthcare were in the mild middle.

Lecanemab and donanemab, monoclonal antibodies for Alzheimer’s, are on the list of drugs in late-stage development that Clarivate analysts will have annual sales of a $1 billion or more in the next five years.

Hospital leaders who have historically viewed price transparency as potentially disastrous can tap into these three tips to leave that mindset behind and capitalize on the opportunities that price transparency provides.