
Keytruda is the first immunotherapy approved for treatment of patients with early renal cell carcinoma after surgery.

Keytruda is the first immunotherapy approved for treatment of patients with early renal cell carcinoma after surgery.
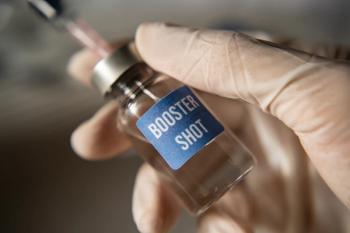
Most of the patients with no immune response after the two-dose regimen responded well to a third shot.

ICER’s review indicates that Humira’s price increases are not supported by new clinical evidence.

If authorized, Paxlovid would be the first oral 3CL protease inhibitor specifically designed to combat the virus that causes COVID-19.
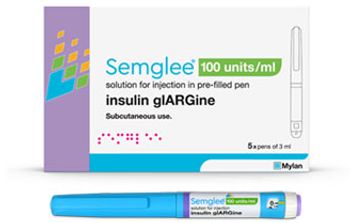
Branded and unbranded versions of the interchangeable biosimilar to treat patients with diabetes are now available.

A reimbursement policy similar to that used for branded and generic drugs would have saved $1.6 billion from 2015 to 2019.

Equipment and process issues could lead to a lack of sterility.


Besremi is the first interferon approved to treat a disease that causes the overproduction of red blood cells.

If approved, Jardiance would be the first treatment for adults across the full spectrum of heart failure regardless of ejection fraction.

Patients with chronic lymphocytic leukemia, non-Hodgkin lymphoma, Hodgkin lymphoma, and multiple myeloma were more likely to have a COVID-19 breakthrough infection.

Smaller PBMs are rated higher by health plans in terms of satisfaction because of their ability to offer more customized solutions.

The safety labeling for the beta interferons that are used to treat multiple sclerosis have been updated to include warnings about injection site reactions.

Previously, the FDA had issued an EUA for those over the age of 65 and those at high risk for severe COVID-19 or exposure to the virus.

Half of the step therapy protocols for specialty drugs evaluated by investigators go beyond what is suggested in treatment guidelines.
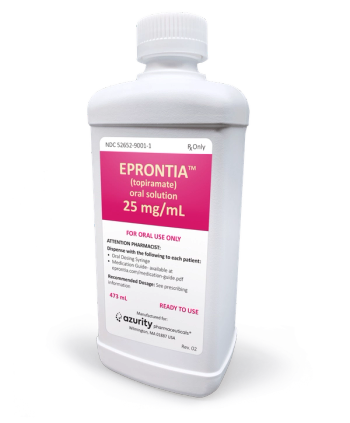
Eprontia is the first liquid formulation of topiramate approved to both treat and prevent seizure.

Paxlovid, an at-home treatment for COVID-19, reduces illness severity, hospitalizations, and deaths among adults by 89%.

CVS Caremark has removed 10 branded products from its formulary and added about 30 additional products.

The studies conducted to support Verzenio’s most recent approval for the treatment of high-risk early breast cancer provided additional safety data.


The FDA didn’t have enough data to assess the risks and benefits of Zyesami.

The antidepressant fluvoxamine reduced the need for hospitalization in high-risk patients, according to a new study.

UK regulatory authorities approved molnupiravir to treat patients with mild-to-moderate COVID-19.

This Tufts study has found that plans that restrict orphan drugs do so by narrowing the patient population who can receive them.

Prime’s formularies will include the insulin biosimilar over the reference product Lantus beginning in January 2022.

The agency needs more time to review new information on the analytical method used.