The warnings section of Inrebic, a JAK inhibitor to treat a bone marrow cancer, now includes information about the cardiovascular risk associated with Xeljanz, another JAK inhibitor to treat arthritis.

The warnings section of Inrebic, a JAK inhibitor to treat a bone marrow cancer, now includes information about the cardiovascular risk associated with Xeljanz, another JAK inhibitor to treat arthritis.
The FDA is reviewing Reblozy to treat anemia in patients with beta thalassemia. The PDUFA action date is March 27, 2022.

CMS is not requiring payment for pharmacists’ testing, patient assessment, ordering/prescribing and dispensing for oral COVID-19 antiviral drugs.

PreHevBrio is a three-antigen hepatitis B vaccine for adults in the United States. It is expected to be available in the first quarter of 2022.

The FDA has set a PDUFA action date of April 1, 2022.

ICER’s first scorecard of the barriers to fair access to medications found that more transparency is needed to fully assess insurers and their coverage policies.

The FDA has assigned a Prescription Drug User Fee Act goal date of Sept. 10, 2022, for deucravacitinib. If approved, it would be the first TYK2 inhibitor approved for the treatment of any disease.

If the FDA authorizes molnupiravir, it would be the first oral anti-viral to treat COVID-19 that patients can take at home.
Findings show substantial variation in payer-negotiated prices and self-pay cash prices at top-performing U.S. hospitals and considerable markups on clinician-administered drugs.

The regulatory agency has set a Prescription Drug User Fee Act date during the first quarter of 2022

The study found that nearly half of Americans diagnosed with high blood pressure do not have it sufficiently controlled.

Merck now says molnupiravir reduces the risk of hospitalization or death from COVID-19 by 30% after all data have been analyzed. Previously, an interim analysis showed a 50% reduction in hospitalization or death.

Moderna and Pfizer are assessing their vaccines against the Omicron variant, which was first identified in South Africa.
Across all payers, the use of generic statins has resulted in a savings of $11.9 billion annually.

New requirements resulted in long call wait times, which has led to patient access issues.

Patients who are part of the Walgreen’s Prescription Savings Club can receive Semglee at up to 80% off the cash price.
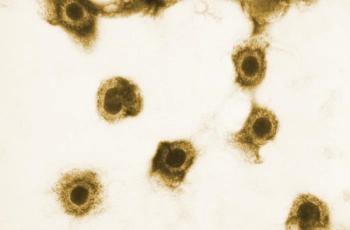
Livtencity is the first therapy approved to treat cytomegalovirus infection, which is common after transplants.
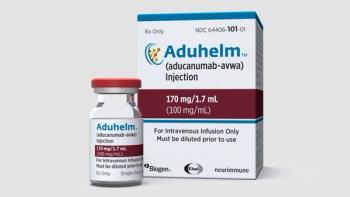
The incidence of brain swelling was highest in the group of patients who received the highest doses of Aduhelm, as well as the group of patients who are carriers of the APoE gene.
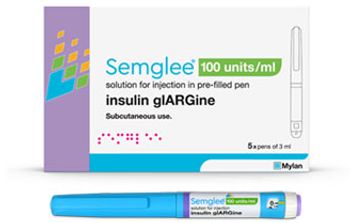
OptumRx will favor the reference product Lantus on the Premium Formulary. The organization also released coverage information about other newly approved therapies.

Several new generics have launched in the United States, including for the migraine therapy Zomig, the anticancer therapy Doxil, and an anti-inflammatory used to treat respiratory conditions associated with COVID-19.
If approved, Bluebird Bio’s beti-cel would be the first one-therapy for β-thalassemia. The PDUFA date is May 20, 2022.

Pfizer/BioNTech plan to use this data to support submissions for full approval of the vaccine in those 12 to 15 years old.

The antiseizure medication affected by the recall could have sterility issues.


Bulevirtide has been granted breakthrough therapy and orphan drug designations by the FDA.