
Apotex's importation of Chantix (varenicline) and Express Scripts' formulary exclusions were among the best read articles this year that didn't concern COVID-19.

Apotex's importation of Chantix (varenicline) and Express Scripts' formulary exclusions were among the best read articles this year that didn't concern COVID-19.

The FDA's warning about Xeljanz (tofacitinib) and other JAK inhiitors and the extension of its review of Janssen's CAR T therapy for multiple myeloma were among the best read articles about the agency in 2021.

The attention-getting articles about COVID-19 included news about Pfizer's booster and the FDA's rejection Zyesami (aviptadil) for treatment of COVID-19-related respiratory failure.

The approval of the generic version of smoking-cessation drug Chantix (varenicline) garnered a lot of attention as did the OKs of Besremi (ropeginterferon alfa-2b-njft), a treatment for polycythemia vera, and Trudhesa (dihydroergotamine mesylate), a nasal spray for acute treatment of migraines.

Reporting of PRO in oncology clinical trials increased after standards from ISOQOL and others were published in 2013.

This is the Areva's second importation of bupivacaine this year.

The FDA has set a Prescription Drug User Fee Act goal date of Sept. 28, 2022.
During the period 2015 to 2020, if a proposed policy that increases Medicaid rebates for drugs with accelerated approvals had been enacted, up to $5.2 billion could have been saved.

Dr. Reddy’s launches generics of Diovan and venlafaxine ER, Apotex launches generic atropine sulfate solution, and Teva launches generic of Epiduo Forte.
Because of a COVID-19 related shortage, facilities may not have immediate access to Actemra, which is required as part of the REMS programs for CAR T-cell therapies.

The broader indication for Zynrelef now covers around 7 million procedures annually and reduces the need for post-surgery opioids.

Consumers have a false sense of security about buying drugs from online platforms.
Blood cancer patients who had at least some antibodies after the first two doses are likely to produce large amounts after the third vaccination.
Researchers suggest that adjusting the prescribing patterns of about 8% of physicians could result in substantial savings.

Medexus is working with Ethypharm to generate the data needed to support an abbreviated new drug application for triamcinolone hexacetonide.

The topical anesthetic was found to be “super potent,” which could lead to systemic toxicity and central nervous system and cardiovascular reactions.
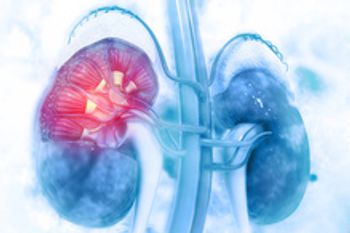
The regulatory agency is reviewing Reata’s application of bardoxolone to treat patients with chronic kidney disease caused by Alport syndrome, a rare genetic disease.

The FDA in late November authorized the COVID-19 booster for all people 18 years of age and older.

Evusheld is the only monoclonal antibody authorized in the United States for COVID-19 pre-exposure prophylaxis.
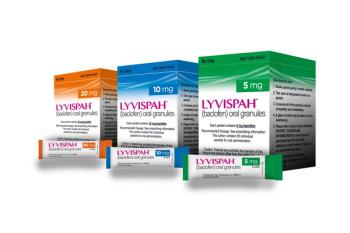
The oral granules formulation allows it to be used with soft foods and with feeding tubes.

Pfizer and BioNTech expect to have an omicron-specific booster ready by March 2022.

Preclinical studies show sotrovimab is active against key mutations of the omicron variant of SARS-CoV-2, which causes COVID-19.

Besremi was approved in November to treat adults with polycythemia vera.

Livtencity, manufactured by Takeda, was approved in November 2021 and is the first therapy to treat cytomegalovirus infection after transplants.
The new boxed warning for Xeljanz, Olumiant, and Rinvoq includes the risk of cardiovascular adverse events and cancer.
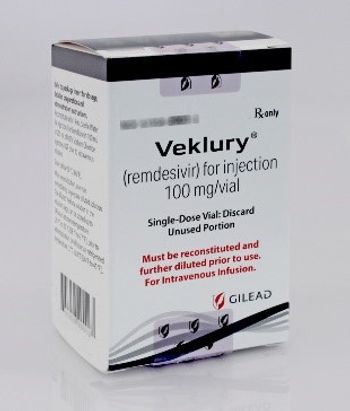
Glass has been detected in the two lots of Veklury, which is used to treat patients who are hospitalized with COVID-19.