
FDA is requiring postmarketing surveillance studies to further assess the risk of myocarditis and pericarditis from the vaccine, which is being sold under the brand name Spikevax.

FDA is requiring postmarketing surveillance studies to further assess the risk of myocarditis and pericarditis from the vaccine, which is being sold under the brand name Spikevax.

The new therapy treats patients with unresectable or metastatic uveal melanoma.
Price increases on promising non–small cell lung cancer drugs despite evidence price competition raise concerns about affordability.
Regeneron Pharmaceuticals and Sanofi said they are voluntarily withdrawing their supplemental Biologics License Application (sBLA) for Libtayo (cemiplimab-rwlc) as a second-line treatment for patients with advanced cervical cancer.

The dual orexin receptor antagonist (DORA) from a Swiss drugmaker will be available in May 2022.

More than $250 million worth of counterfeit and illegally resold versions of Gilead Sciences’ HIV medications Biktarvy (bictegravir, emtricitabine and tenofovir alafenamide) and Descovy (emtricitabine and tenofovir alafenamide) were distributed to pharmacies and patients.
Experts says Regeneron and Eli Lilly’s antibody treatments won’t work against omicron, but Florida Gov. Ron DeSantis calls the FDA’s decision to pause the emergency use authorization “just wrong.”

The new Medi-Cal Rx program that debuted Jan. 1 has alarmed health clinics that say the state's Medicaid will lose money and have to cut services.

The company joins the wide array of online pharmacy services, such as Amazon Pharmacy, that have popped up in recent years. Cuban has also launched a namesake PBM.

The Janssen drug is the only DOAC available in an oral suspension, which facilitates pediatric weight-based dosing.
United States healthcare spending rose 9.7% in 2020, while medication spending is increasing by more than 18%.
The limit is four tests per household.

The FDA okayed two Janus kinase 1 (JAK1) JAK inhibitors January 14 the for atopic dermatitis: Cibinqo and Rinvoq.

In a research letter published in JAMA Health Forum, researchers say most of the cost figures for Aduhelm have left out the additional MRI scans and other services that will be required because the drug is associated with the development of amyloid-related imaging abnormalities (ARIAs).
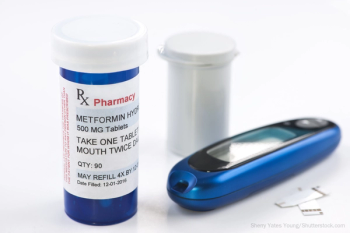
Problems with N-nitrosodimethylamine (NDMA)-contaminated metformin were identified by the FDA in 2020. Viona Pharmaceuticals announced in earlier this month that it was recalling 23 lots of the popular diabetes medication.
Leo Pharma's Adbry (tralokinumab-ldrm) is the second, FDA-approved biologic for atopic dermatitis. Dupixent (dupilumab) was the first.

CMS has proposed covering the anti-amyloid treatment only if the person taking it is enrolled in a clinical trial.
Smaller doses are recommended for people with moderate renal impairment and the treatment should not be taken by people with serious kidney disease. There are also drug interactions to be aware of because Paxlovid is an inhibitor of CYP3A.

Lecanemab and donanemab, monoclonal antibodies for Alzheimer’s, are on the list of drugs in late-stage development that Clarivate analysts will have annual sales of a $1 billion or more in the next five years.


Leqvio (inclisiran) is the first small interfering RNA therapy approved to lower LDL cholesterol in certain adults at risk for life-threatening cardiovascular events.
But Paxlovid and molnupiravir will be available only at certain Walmart and Sam’s Club locations.


A review of real-world data from Israel didn't show any increase risk of myocardities or pericarditis.
ICER's analysis is slated to include two as-yet unapproved monoclonal antibody treatments, donanemab and lecanemab, as well as Aduhelm (aducanumab).