
The recall of Accuretic and generics distributed by Greenstone is a result of the presence of a cancer-causing agent.

The recall of Accuretic and generics distributed by Greenstone is a result of the presence of a cancer-causing agent.

The FDA also approved a companion diagnostic to select patients with mismatch repair deficiency who would be eligible for treatment with Keytruda.
But there is no significant difference in rate of hospitalization for major bleeding when Eliquis is compared with Xarelto or warfarin.

Opdualag is a first-in-class immunotherapy that combines Opdivio with the novel LAG-3-blocking antibody relatlimab.

Teplizumab is being evaluated to delay type 1 diabetes. The PDUFA date is August 17, 2022.

Ztalmy was approved for seizures associated with CDKL5 deficiency disorder in patients two years of age and older. It is expected to be available in July 2022.

Del Doherty, co-founder of Prodigy Rx, discusses how the PBM aims to give payers control so they can lower costs and can improve clinical and return-to-work outcomes.

Amneal is of 35 global companies selected to manufacture and commercialize generic version of COVID-19 treatment Paxlovid.
New data show longer-term benefits from Aduhelm, which was approved last year to treat Alzheimer’s disease.

ICER analyzed two therapies — Cosela and plinabulin — to prevent chemotherapy-induced neutropenia and other myelosuppressive effects. Both moderately increased quality-adjusted life-years.

Allan Coukell, Civica’s senior vice president of public policy, discusses how the generic manufacturer is disrupting the market for insulins.

Evio Pharmacy Solutions plans to provide biosimilar alternatives for autoimmune and cancer therapeutics.

Called Breyna, this drug-device combination generic product is a metered-dose inhaler, which contains both budesonide and formoterol.

In an interim analysis, Keytruda/Lynparza did not improve overall survival in patients with metastatic castration-resistant prostate cancer who progressed after treatment with chemotherapy.
After a phase 3 trial showed that Cabometyx/atezolizumab did not improve overall survival in patients with hepatocellular carcinoma, Exelixis officials have said they won’t be submitting an NDA for untreated patients with advanced liver cancer.

The FDA has asked for clinical data for Fasenra, which is being reviewed as a treatment for chronic rhinosinusitis with nasal polyps.
Adlarity delivers donepezil to patients using a skin patch, lowering the risk of gastrointestinal adverse events. It will be available in early fall 2022.
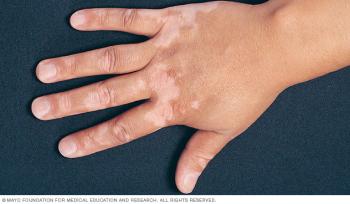
Opzelura is being reviewed as a treatment for vitiligo, a disease that causes the loss of skin color. The new PDUFA date is July 18, 2022.
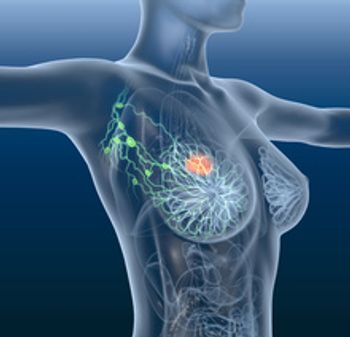
Lynparza reduced the risk of death by 32% versus placebo. It is the first targeted treatment for patients with early breast cancer to be used after surgery or prior treatment.

The lots could contain trace amounts of sodium, silicon, chromium, aluminum, and cellulose, which could result in irritation or infection and has the potential to block blood vessels in the heart, lungs, or brain.

The FDA has granted first approval for cartridges for use with Apokyn for Parkinson’s. Other approvals include: a generic of anticancer therapy Vidaza, the antibacterial Erythrocin, and the anti-epilepsy therapy Lamictal.

B. Braun is recalling five lots because of fluid leakage, which could expose patients to a bacterial or fungal infection.
This expands commercial coverage to 118 million lives and Medicare coverage to 7.1 million lives.

The company will distribute nalmefene for no profit, which is part of the company’s bankruptcy filing and settlement with states.

Supplemental applications have been submitted for the cancer therapies Nubeqa and Tibsovo, the gout treatment Krystexxa, and Oxlumo, which treats a rare disease.