Laurie Sobel, associate director for women’s health policy at KFF, moderated a panel discussion about the challenges associated with providing insurance coverage for the first FDA-approved oral contraceptive, Opill.

Laurie Sobel, associate director for women’s health policy at KFF, moderated a panel discussion about the challenges associated with providing insurance coverage for the first FDA-approved oral contraceptive, Opill.
An analysis from 3 Axis has found that PBMs control how much pharmacies are reimbursed. This can vary significantly depending on the PBM contracts with insurers, creating a system with large inconsistencies in the prices that consumers pay for both generics and branded products.

The FDA has requested an additional study. ARS Pharmaceuticals plans to appeal the decision.

Atidarsagene autotemcel is a one-time gene therapy to treat patients with the rare and fatal disease metachromatic leukodystrophy (MLD). The agency has set a review date of March 18, 2024.
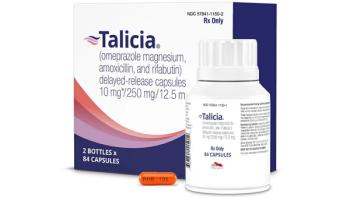
Earlier this year, RedHill Biopharma began a warranty program for patients taking Talicia, with a commitment to reimburse patient out-of-pocket costs if Talicia does not work.
Momelotinib — now with the brand name Ojjaara — is the first treatment for myelofibrosis patients with anemia. It has a list price of $26,900 for a bottle of 30 tablets.
Even though committee members voted in support of Onpattro for patients with cardiomyopathy related to transthyretin-mediated amyloidosis, there were questions about whether it provided a clinically meaningful benefit. The FDA set an action date of Oct. 8, 2023.
The date has been extended by three months to Feb. 24, 2024, because of resource constraints at the regulatory agency.
CMS has included an additional 34 drugs on the list on products subject to rebates if their price increases are higher than inflation.

This simulation study also showed that semaglutide was the most effective for weight loss, and researchers said, long-term, savings associated with potential lifelong health improvements may shift the balance of cost-effectiveness.
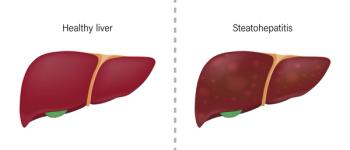
The FDA has granted priority review and assigned a review date of March 14, 2024, for resmetirom treat adult patients with nonalcoholic steatohepatitis (NASH) who have liver fibrosis.

Cell and gene therapies can be used for patients with cancer and serious diseases who have limited or no treatment options — but at a cost.

Addressing drug affordability and accessibility will involve tackling the interrelated problems of high prices and problematic pharmacy benefit manager practices, says Benjamin N. Rome, M.D., with Brigham and Women’s Hospital in Boston.
Miebo, approved in May, addresses tear evaporation in dry eye disease. It will have a wholesale acquisition cost of $771 for a one-month supply.
In clinical trials, Aphexda plus filgrastim enabled a majority of patients to achieve the collection goal to enable stem cell transplantation for patients with multiple myeloma. It will have a list price 5,900 per vial, with most patients needing two vials for treatment.

In total, Medicare beneficiaries paid $21 billion for 100 drugs that receive the most rebates, while health plans paid $5.3 billion after rebates.
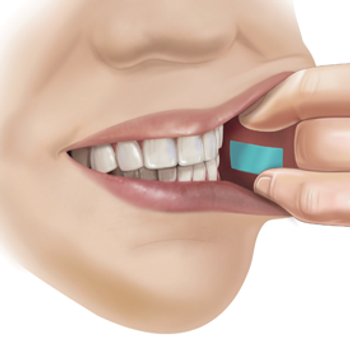
Libervant is film version of diazepam that is placed inside the cheek to treat children between two and five years of age who have refractory epilepsy. The PDUFA target goal date is April 28, 2024.

Crystal formation could lead under- or over-dosing of Sandimmune oral solution, which is used to prevent organ rejection in kidney, liver, and heart allogeneic transplants.

This House bill combines aspects of previously submitted bills — specifically on transparency related to prices on hospital services, laboratory tests, healthcare coverage and PBMs — into one package.

The decision by Scripius (previously Select Health Prescriptions) was made based on the cost of Dupixent, as well as because of an increased demand to use Dupixent for mild atopic dermatitis when the primary patient population is those with moderate to severe disease.

In a 10-year, follow-up study in patients vaccinated with Gardasil 9, no cases of HPV-related cancers or genital warts were seen.

If approved, crovalimab will be the first monthly subcutaneous treatment for patients with paroxysmal nocturnal hemoglobinuria, a rare, life-threatening condition.

If approved, mavorixafor would be the first therapy to address the genetic defect that results in WHIM syndrome, an ultra-rare disease that can cause recurrent lung infections, papillomavirus-related warts, and an increased risk of developing certain types of cancer.
With this tentative approval of abacavir/dolutegravir/lamivudine, Viatris aims to help adherence to HIV treatment in low- and middle-income countries.

Magellan Rx Management has begun providing a multi-state solution in which they negotiate with drug manufacturers for value-based contracts for high-cost gene and cell therapies.

For most drugs, the statutory minimum discounts under the Inflation Reduction Act will not be enough to achieve the savings projected by the Congressional Budget Office.