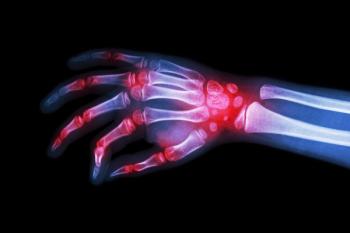
Patients who continued with their rheumatoid arthritis biologic medication tended to spend less on healthcare, had a lower chance of being hospitalized, and had shorter hospital stays.

Patients who continued with their rheumatoid arthritis biologic medication tended to spend less on healthcare, had a lower chance of being hospitalized, and had shorter hospital stays.


Issues related to finding from an FDA inspection at a third-party filler have been resolved.

Mark Campbell, Pharm.D., RxBenefits’ senior vice president of clinical solutions, talks about how Medicare’s drug price negotiations could have a ripple effect on pharmaceutical company innovation and prices on the commercial side.
Betaxolol may cause a slowing in the heart rate in some patients; an opioid could increase the effect.
Galderma officials said they have already identified changes to its manufacturing process for relabotulinumtoxinA, which is being reviewed as a treatment for frown lines and crows feet.

ICER has weighed in on two of the 10 drugs selected for negotiations: Eliquis and Xarelto, which are among Medicare Part D’s highest spending drugs.

Once Medicare’s negotiated price for prescription drugs goes into effect, payers have more of an incentive to steer patients to lower-cost alternatives.

In a confirmatory trial, Exkivity did not meet the primary endpoint in treating patients with non-small cell lung cancer with EGFR exon 20 mutations. It will remain available while Takeda works with the FDA on withdrawal timing.
FDA has set an action date of March 31, 2024, for marnetegragene autotemcel to treat infants with a serious and often fatal immunodeficiency.
Biogen is still evaluating launch timeline for Tofidence, and information on pricing will be available closer to launch.

Aetna, Cigna, Humana, United Healthcare — some of the largest providers of Medicare Advantage plans — have released updates to prescription drug programs.
At issue are findings from the agency’s inspection of a third-party manufacturing company. The FDA indicated there were no concerns about the safety or labeling of lebrikizumab.

The two-component therapy of Pombiliti plus Opfolda to treat adults living with late-onset Pompe disease will have an annual list price of $650,000.
Odronextamab is a bispecific antibody to treat relapsed/refractory follicular lymphoma and diffuse large B-cell lymphoma. The target action date is March 31, 2024.
The recent study also found that Jardiance for patients with heart failure with preserved ejection fraction could be cost-effective if discounted by 29%.

Contamination of Brexafemme with a non-antibacterial beta-lactam drug could lead to reactions such as swelling, rash, urticaria and anaphylaxis.

Exxua is approved to treat adults with major depressive disorder, but its labeling does not contain warnings about sexual function or weight gain.
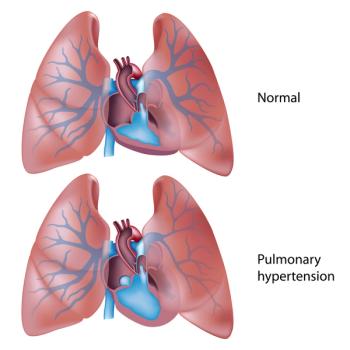
Sotatercept is a first-in-class therapy to treat the rare disease pulmonary arterial hypertension. The FDA has assigned a target action date of March 26, 2024.

The class action lawsuit against CVS Health, Caremark, and Aetna was announced earlier today.

Ryzumi is expected to be available in the first half of 2024. Pricing will be available around the time of launch.

Svetlana Mojsov, Ph.D., was a key part of the team that discovered the function of the hormone glucagon-like peptide 1 (GLP-1) and its role in diabetes and weight loss.
Iyuzeh can be stored at room temperature. It has a list price of $299 for a month’s supply.
The FDA has assigned a target action date of Jan. 31, 2024, for Dupixent to treat children 1 to 11 years of age with the inflammatory condition eosinophilic esophagitis.

In patients who are immunocompromised, Bacillus cereus can result in life-threatening infections such as endocarditis and necrotizing soft tissue infections.

ICER adds a more formal process to evaluate the diversity of clinical trials and an assessment of a product’s impact on patient and caregiver productivity. ICER also plans to evaluate how newer methods — which would consider the change of a drug’s price over time and disease severity — can be applied to its value assessment.