Uro-Oncology
Latest News
CME Content


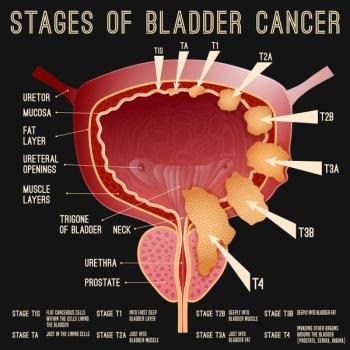
Researchers in China combined findings from MRI scans with clinical features to create a new predictive model for nonmuscle-invasive bladder cancer recurrence.

The PIVOT-006 trial is part of a larger national effort to evaluate cretostimogene’s safety and effectiveness in bladder cancer.
The FDA approved the system in September 2025.
Recently reported results from an open-label extension trial show positive safety and efficacy results after 52 weeks of use of the drug for overactive bladder.
Blujepa is the first in a new class of oral antibiotics in 30 years. It is expected to launch in the second half of 2025 in the United States.

Spero Therapeutics was seeking approval for tebipenem HBr oral tablets for treatment of adult patients with complicated urinary tract infection. The FDA has requested an additional clinical trial.

Approved in March, Tlando is an oral testosterone that doesn’t require dose titration.
Livmarli is the first drug approved for Alagille syndrome.

Recent FDA action (through December 2012) related to, Rabeprazole sodium delayed-release sprinkle capsules, CXA-201, CB-215, Buprenorphine subdermal implant, LX1033, ISIS-TTR Rx, Digoxin Immune Fab, Glucagon, AAV1-FS344, Trans sodium crocetinate, Betamethasone valerate foam 0.12%

Recent FDA Approvals (through December 2012) related to (Bedaquiline, Janssen, Oseltamivir, Genetech, Apixaban, Bristol-Myers Squibb, Lomitapide, Aegerion, Varicella zoster immune globulin preparation, Cangene, Teduglutide, NPS Pharmaceuticals, Pasireotide diaspartate, Novartis, Ponatinib, ARIAD Pharmaceuticals, Influenza virus vaccine, GlaxoSmithKline, Raxibacumab, Crofelemer, Salix, Cabozantinib, Exelixis)

New molecular entity: FDA approved the soral phosphodiesterase-5 (PDE-5) inhibitor, avanafil (Stendra, Vivus) for the treatment of ED.

Recent FDA action (through June 2012) related to, Darunavir, Prezista, Janssen Therapeutics, Ridaforolimus, Merck, Tafamidis meglumine, Pfizer, mechlorethamine, Ceptaris Therapeutics, AVP-923, Avanir Pharmaceuticals, NKTR-181, Nektar Therapeutics, EDI200, Edimer Pharmaceuticals, Galeterone, TOK-001, Tokai Pharmaceuticals, Butoconazole nitrate 2% vaginal cream, Gynazole-1, Perrigo

Recent FDA action (through May 2012) related to, Loxapine, Adasuve, Alexza Pharmaceuticals, Denosumab, Xgeva, Amgen, Furiex, Takeda, Nesina, alogliptin, fixed-dose combination alogliptin and pioglitazone, Liovel, Emtricitabine and tenofovir disoproxil fumarate, Truvada, Gilead Sciences, Lorcaserin, Arena Pharmaceuticals, Eisai, Tofacitinib, Pfizer, Tafamidis, ACH-3102, Achillion, CK-2017357, Cytokinetics, Taliglucerase alfa, Elelyso, Pfizer, Carisbamate, SK Biopharmaceuticals, Vancomycin hydrochloride, Vancocin, Akorn, Watson

Recent FDA action (through April 2012) related to, Dihydroergotamine, Levadex inhalation aerosol, droxidopa, Northera, Amitriptyline 4% ketamine 2% cream, AmiKet, Afilbercept, ZALTRAP, Nav rAAV8 vector, Carbamazepine, Carbatrol, Vancomycin hydrochloride, Vancocin, Irbesartan, irbesartan-hydrochloriderothiazide, Avapro, Avalide, Fluvastatin, Lescol

Recent FDA action (through February 2012) related to C1 esterase inhibitor [human], progesterone vaginal gel 8%, meningococcal [Groups A, C, Y, and W-135] oligosaccharide diphtheria CRM197 conjugate vaccine, denosumab, VAL-083, recombinant fusion protein linking coagulation factor VIIa with albumin, pertuzumab in combination with trastuzumab, crofelemer 125-mg tablets, emtricitabine/tenofovir disoproxil fumarate, tafamidis meglumine, synthetic version of the natural human hormone secretin, doxycycline hyclate

Recent FDA Approvals (through December 2011) related to (Antares, Isentress, REMS)

New indication: Tadalafil once-daily 5-mg oral tablet is FDA approved for the treatment of benign prostatic hyperplasia (BPH) in patients with or without erectile dysfunction (ED).

The 5-alpha-reductase inhibitor dutasteride is associated with markedly lower BPH-related complication rates than the alpha-blocker tamsulosin, according to analysis of 2 large trials.

The selective beta3-adrenoceptor agonist mirabegron effectively improves symptoms of overactive bladder and is very safe and well tolderated, according to results of a phase 3 study.

Patients with metastatic castration-resistant prostate cancer treated with the investigational agent abiraterone acetate plus low-dose prednisone/prednisolone showed a significant improvement in overall survival compared to patients treated with prednisone/prednisolone plus placebo, according to a phase 3 study published in the May 26 issue of the New England Journal of Medicine.

Generic drugs approved by FDA (through May 2011): bromfenac ophthalmic solution 0.09%, sodium ferric gluconate complex in sucrose injection, nitrofurantoin oral suspension

Denosumab (Xgeva, Amgen) significantly increased bone metastasis-free survival for more than 4 months in men with castrate-resistant metastatic prostate cancer that had not yet spread to bone, according to results of a landmark study of Xgeva, presented at a late-breaking plenary session at the American Urological Association?s 2011 annual meeting in Washington, DC.