Relmada Therapeutics appoints Max Kates to clinical advisory board to support NDV-01 phase 3 program for bladder cancer
Key Takeaways
- Relmada Therapeutics is advancing NDV-01, a novel intravesical therapy for NMIBC, into phase 3 clinical trials, with Max Kates, M.D., joining their advisory board.
- NDV-01 combines gemcitabine and docetaxel in a sustained-release formulation, aiming to improve efficacy and tolerability by providing prolonged tumor exposure while minimizing systemic toxicity.
Kates, a nationally recognized expert on bladder cancer, lends credibility to the company’s efforts to develop a new treatment for bladder cancer.
A nationally recognized expert in bladder cancer, Kates currently is an associate professor of urology and oncology at the Johns Hopkins University School of Medicine, where he also serves as the R. Christian B. Evensen professor of urology and director of the Division of Urologic Oncology.
In his roles, he oversees one of the largest surgical bladder cancer practices in the country and has led or contributed to several landmark clinical trials. Kates’ work bridges clinical practice and translational research, with a focus on improving outcomes for patients with NMIBC and muscle-invasive disease.
The Coral Gables, Florida-based company noted that his appointment will help guide the design and execution of its upcoming phase 3 trial of NDV-01, with plans to begin the study in the first half of 2026.
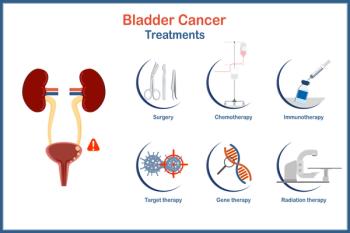
NDV-01 is a sustained-release, intravesical formulation of the chemotherapy combination gemcitabine and docetaxel, designed to provide prolonged exposure to tumor sites while limiting systemic toxicity. The formulation can be administered in a urology office setting in less than 10 minutes without anesthesia, simplifying the treatment process for both patients and clinicians.
According to Relmada, bladder cancer is the 10th most common cancer globally, and NMIBC accounts for approximately 75% to 80% of all cases. Despite a generally favorable prognosis, recurrence rates remain high, estimated at 50% to 80% within five years, creating a major clinical and economic burden. In fact, many patients ultimately progress to muscle-invasive disease, which is associated with poorer outcomes and more aggressive interventions.
Relmada’s NDV-01 aims to fill a significant therapeutic gap, particularly in the setting of Bacillus Calmette-Guérin (BCG)-unresponsive or recurrent NMIBC, where treatment options are limited. The company believes that by combining two well-known chemotherapeutic agents in a novel sustained-release matrix, NDV-01 can achieve higher local concentrations in the bladder and potentially improve both efficacy and tolerability compared with conventional intravesical regimens.
Kates’ experience will be instrumental in shaping the NDV-01 program as it advances toward pivotal testing. His insights into patient selection, trial endpoints, and surgical coordination are expected to ensure that the upcoming study aligns with real-world practice and reflects current standards of urologic oncology care.
Kates is also known for his leadership in the national phase 3 BRIDGE (EA8212) trial, evaluating alternative bladder-sparing strategies in NMIBC. His participation in Relmada’s CAB underscores the company’s commitment to incorporating input from leading clinician-scientists at the forefront of bladder cancer research.
The planned phase 3 study of NDV-01 will evaluate the candidate’s efficacy and safety as a potential frontline or salvage therapy across NMIBC subtypes. Relmada intends to finalize trial design and regulatory engagement in the upcoming months, building on encouraging preclinical and early clinical data that demonstrated prolonged bladder retention of the therapy and strong tolerability signals.
The company’s broader strategy involves expanding its oncology pipeline and integrating clinical expertise through its advisory board to help bring new, practical, and effective options to patients with non-muscle invasive bladder cancer.
Newsletter
Get the latest industry news, event updates, and more from Managed healthcare Executive.