NSCLC
Latest News

Latest Videos

CME Content
More News
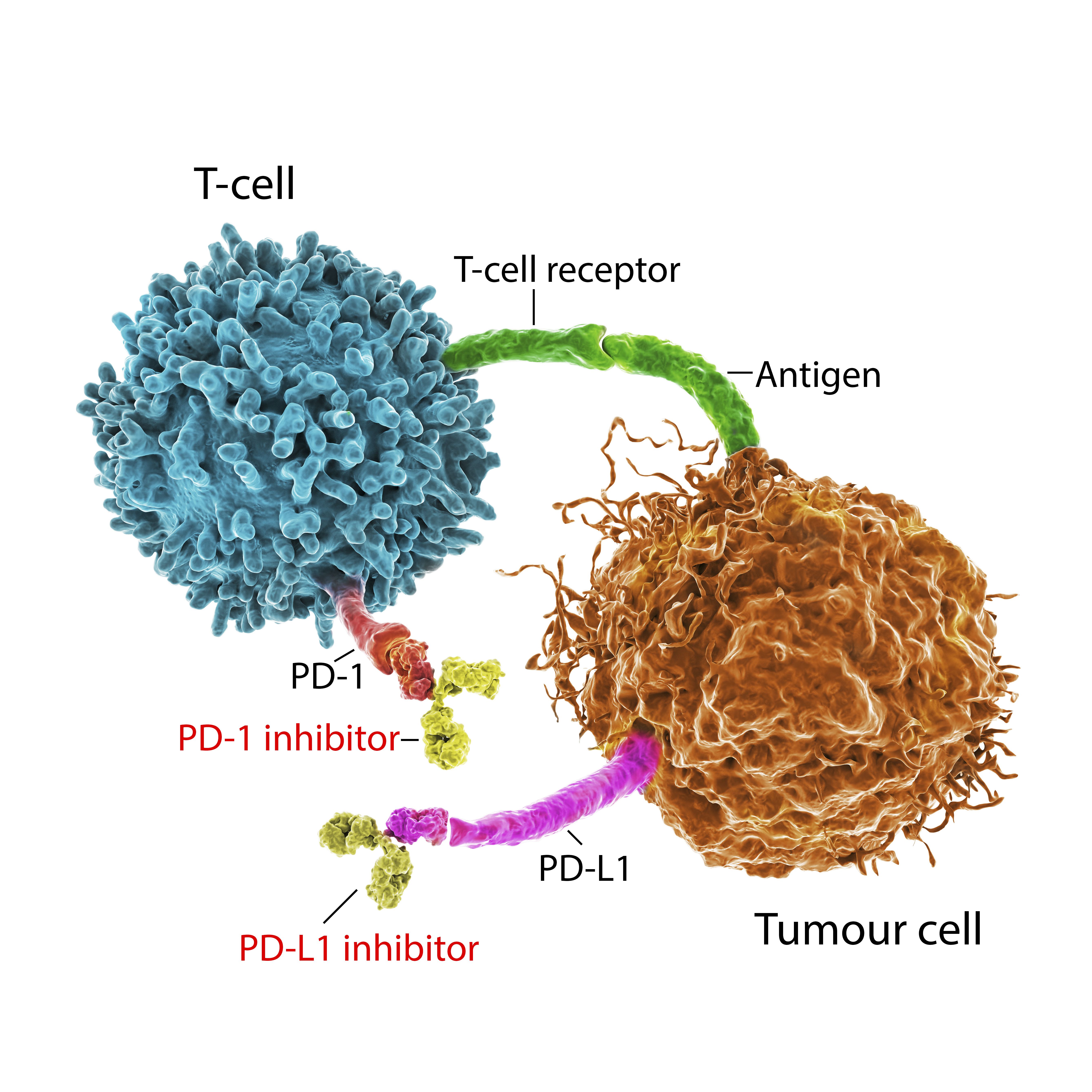
Newly approved drugs put the brakes on particular “driver mutations,” that when unimpeded, rev up the runaway cell growth and division that characterizes cancer.
Patients with high amplification of the MET gene had a response rate of 38.1%, more than twice the response rate of those with medium amplification.

Study shows that nonsmall cell lung cancer patients who smoke but quit after their diagnosis have better overall survival, progression-free survival than patients who continue to smoke.
When wondering what step to take first after learning of your cancer diagnosis, assembling the right care team and creating a treatment plan designed uniquely is important to get you on the right track. Comprehensive biomarker testing can then help identify your type of lung cancer and help move steps forward for proper care.
Sands ,a medical oncologist who specializes in lung cancer, says the false discovery has been misconstruted as a false positive rate. He also makes a case for early detection and new USPSTF as working to correct disparities.
If the preliminary findings are validated in larger studies, the method could help clinicians determine which patients are candidates for immune checkpoint inhibitor therapy.
Positive findings for Tepmetko and Tabrecta reported at ASCO.
The FDA approved Amgen’s Lumakras (sotorasib) last week, the first drug targeting KRAS-mutated cancer to get the agency’s OK. But the clinical and commercial future of the drug is uncertain.
Meta-analysis shows lower prevalence of four known driver mutations among Black lung cancer patients. More research into tumor biology of Black patients might yield targeted therapies that would benefit Black lung cancer patients, says the lead author, Philippos A. Costa, M.D.

Patients who were PD-L1 positive with early stage non-small-cell lung cancer had better results and a lower risk of death or progression compared to a cohort on best supportive care.
The FDA gave its first approval today of a cancer drug that targets a KRAS mutation.

Precision medicine is getting that much more precise. Today's FDA go-ahead is for a J&J drug that is designed to treat the 2% to 3% of NSCLC patients wiht EGFR exon 20 insertion mutations.

CVS Health research shows $7,084 cost disadvantage to care that is not "concordant" with National Comprehensive Care Network guidelines.

A small fraction of those who were eligible under previous U.S. Preventive Services Task Force recommendations were screened with low-dose CT scans. New recommendations will make an additional 6.4 million Americans eligible, but a number of barriers to screening remain.

The prevalence of smoking among African American young adults “catches up” with other groups despite the low rate among Black youth. The ban on menthol may affect this and other aspects of smoking patterns among Black Americans.

Chemo in combination with immunotherapy was preferable to immunotherapy alone in patients with a mutation of the KRAS gene and high levels of programmed cell death ligand 1, according to findings reported in JAMA Oncology.
FDA has recently approved therapies targeting MET exon 14 skipping mutations.

Biomarker status can help clinicians and patients make informed, personalized treatment decisions, whether the choice is an FDA-approved targeted therapy or a clinical trial.

Diagnoses spike in people's 65th year when most Americans become newly eligible for Medicare coverage.
Although smoking is the predominate risk factor for lung cancer, about 1 in 5 cases cases are believed to be linked to genetic factors.
City of Hope researchers find longer overall survival rates among nonsmall cell lung cancer patients (NSCLC) who are immigrants. Could this be another instance of the healthy immigrant paradox?

The U.S. Preventative Services Task Force recently released an updated recommendation addressing lung cancer screening criteria at a sooner rate starting at 50-years-old rather than 55. The task force says this expansion will be especially helpful to those who face disparities to screening. Although, it won’t cover all disparities.

Population-level study of Medicare beneficiaries shows marked improvement in survival statistics but also an 8.6% jump in Medicare spending.

Based on results of the VISION study, the FDA has granted accelerated approval to tepotinib for MET exon 14 skipping altered metastatic non–small cell lung cancer.

The pandemic has led to the adoption of telehealth and greater access to care through apps.