Diabetes
Latest News
Latest Videos

CME Content
More News

Byetta is used to control blood sugar in patients with diabetes. A new warning includes the risk of gallstones and gallbladder inflammation.
If insulin copays were capped at $35, Part D enrollees would save 29% on average, according to new analysis from Kaiser Family Foundation.

Mounjaro is a first-in-class medicine that activates both the GLP-1 and GIP receptors, which leads to improved blood sugar control.
Ozempic is now available in three doses to improve blood sugar in people with type 2 diabetes.

Allan Coukell, Civica’s senior vice president of public policy, discusses how the generic manufacturer is disrupting the market for insulins.
Once approved, the insulins will be available to patients for no more than $30 per vial.

The cost-effectiveness group say Lilly’s diabetes treatment should have an annual price of between $5,500 and $5,700. The FDA is expected to make a decision on whether to approve tirzepatide this year.

Patients who are part of the Walgreen’s Prescription Savings Club can receive Semglee at up to 80% off the cash price.
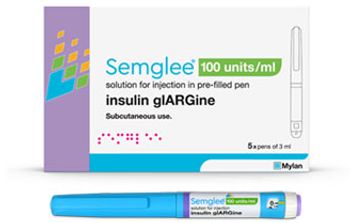
OptumRx will favor the reference product Lantus on the Premium Formulary. The organization also released coverage information about other newly approved therapies.

Branded and unbranded versions of the interchangeable biosimilar to treat patients with diabetes are now available.
At a placeholder cost equal to Ozempic, tirzepatide scored lower in a measure of cost-effectiveness compared with Ozempic but higher than Jardiance.

Prime’s formularies will include the insulin biosimilar over the reference product Lantus beginning in January 2022.

Express Scripts will put the first interchangeable biosimilar insulin on its National Preferred Formulary and exclude Lantus, the reference product.
Effective Jan. 1 2022, the move brings the price to 2008 levels.
FDA approval means that pharmacists can swap biosimilar Semglee, an insulin product, for brand-name Lantus, although state-level pharmacy rules may apply.

The approval allows for substitution at the pharmacy counter for Lantus, its reference product.

Keytruda gets a full approval in endometrial cancer; while Xeljanz will miss another PDUFDA date.

New vaccine for pneumococcal disease, an sNDA approved based on real-world data, a new therapy for skin and muscle disease, Keytruda combination receives full approval in endometrial cancer, a new diabetes therapy is approved, and another JAK inhibitor misses PDUFA date round out this week’s FDA news.

FDA staff raised safety concerns ahead of the advisory panel hearing.

Keytruda loses one indication but gains another, Biogen narrows use of Aduhelm, Padcev gets regular approval and expanded indication, Amgen submits application for asthma biologic, agency issues complete response letter for diabetes prevention therapy teplizumab, Novartis resubmits NDA for inclisiran.

Study published in the Annals of Internal Medicine elaborates on past positive findings for Zynquista (sotagliflozin), a dual inhibitor of SGLT1 and SGLT2.
A longstanding four times per day testing requirement will cease, and patients using inhaled insulin now qualify for continuous glucose monitoring.
Semaglutide injection is a weekly injection for chronic weight management in adults.

Harvard researchers say, well, maybe.
The AstraZeneca drug is the first SGLT2 inhibitor approved for chronic kidney disease regardless of whether the patient has diabetes.