
The PDUFA date for Alkermes' combination therapy is November 15, 2020.

The PDUFA date for Alkermes' combination therapy is November 15, 2020.

Can data mined from EHRs match that from the gold standard, randomized clinical trials?

Approval for treatment of HES, update to address addictive nature of benzodiazepines.

Reports say Azar is asserting control over FDA as vaccine trials near completion, while Hahn is blocked from testifying on COVID-19.

A session at MSVirtual2020 covers the difficulty of finding treatments for some forms of MS.
The 2020 meeting, presented in a virtual format from its original host city of Amsterdam, The Netherlands, had lots of important science and a record number of viewers.

But there are plenty of holdouts. Half of submissions are still done by phone or fax.

Dovato lets adults with HIV reduce the number of antiretroviral therapies they take, while maintaining efficacy and a high barrier to resistance comparable to tenofovir-based regimens.

The American College of Cardiology issues its second set of recommendations for its members on prescribing two groups of drugs first developed for type 2 diabetes.

Lives can be saved, physician burnout and waste can be reduced, and drug costs can decrease by achieving medication optimization through comprehensive medication management.

Advocates say a number of regulatory changes could help bring more of these products on to the market.

Heart disease drugs were in the news this week.

Drug development in key areas of unmet need hit milestones this week, with Biogen, AstraZeneca, and Jaguar Health announcing regulatory steps with the FDA.

FDA outlines what it will take to get a COVID-19 vaccine approved, while NIH offers its research strategy.
FDA approves Merck's Keytruda for certain patients with metastatic colorectal cancer a month after study results were presented at ASCO.
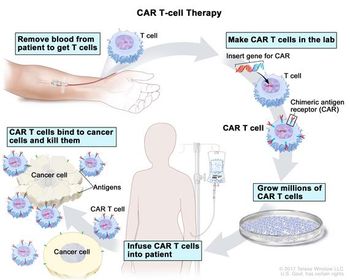
An off-the-shelf version of CAR-T therapy that uses the immune system’s natural killer cells is a possibility. But the high price of CAR-T shows some doubts about how widely it can be used.

New plan from CVS Caremark eliminates member cost as a barrier to medications without raising costs for benefits, premiums or deductibles.

Novel drugs, value-based contracting, biosimilars, and regulatory activity addressing costs will be among the hot button issues in the coming year.

In these arrangements, drug companies must partially reimburse insurers if the drug fails to earn its keep. Will COPD drugs be included in such arrangements?

Despite a scolding from President Trump over drug price hikes, drug giant Pfizer announced it will raise the prices of 41 medications in January. That’s 10% of its entire drug portfolio.



Albert A. Rizzo, MD, senior medical advisor for the American Lung Association, says the increasing number of drugs in the pipeline could improve airflow and address the underlying disease process.

External, non-payer generated data can inform formulary decision-making-if payers know their strengths and weaknesses.

Recently, FDA Commissioner Scott Gottlieb announced the FDA will move to close loopholes in the Orphan Drug Program. Here’s what you need to know.