
Researchers in Switzlerland reported results from a small, proof-of-concept study in JAMA Ophthalmology that suggest an iPhone 11 and an app may be superior to traditional measurement with an Hertel exophthalmometer.

Peter Wehrwein has been the lead editor of Managed Healthcare Executive since February 2020.

Researchers in Switzlerland reported results from a small, proof-of-concept study in JAMA Ophthalmology that suggest an iPhone 11 and an app may be superior to traditional measurement with an Hertel exophthalmometer.

The National Alliance of Healthcare Purchaser Coalitions issued a report today debunking what it says are 10 myths used by hospitals to justify the high prices they charge health plans sponsored by employers. The American Hospital Association responded with a statement that said report was a rehash of anti-hospital talking points.

Patient autonomy needs to be balanced with an obligation to educate patients about the risk and benefits of the complementary and alternative medicine treatments, say the authors of an article in the Journal of the American Academy of Dermatology.
Injectafer (ferric carboxymaltose injection) was no better than a placebo in a double-blind study of patients with heart failure with reduced ejection fraction. The researchers offered several possible explanations for the unexpected result. Were reduced hospitalizations during the COVID-19 pandemic perhaps a factor?

The GLAAD HIV Stigma Survey also showed big differences in HIV knowledge among generations.
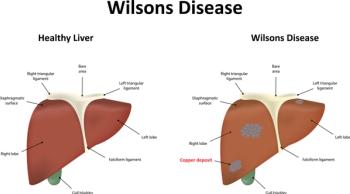
Two gene therapy trials using adeno-associated viral vectors are underway. A review paper published recently in the New England Journal of Medicine says lifelong treatment with chelators is effective but adherence is a problem.

Thompson, the president of the National Alliance of Healthcare Purchaser Coalitions, said if that 20% of the market changes way it deals with PBMs “it will change the market, it will change the conversation, it will change the practices of key players.”

The advent of the direct-acting antivirals, such as Harvoni (ledipasvir and sofosbuvir) means people can be treated for HCV infection if they receive a heart from an HCV-viremic donor, according to a recent review paper. The supply of hearts available for transplantation has increased, partly because HCV-viremic individuals are now part of the donor pool.

Panelists at the 2023 PBMI Annual National Conference mentioned post-marketing studies and changing the mind-set about what a prescription entails.

Mike Steelman, vice president, Managed Markets, Dexcom presented on the pathways to implement non-prescription digital therapeutics at the 2023 PBMI Annual National Conference in Orlando. In this interview with Managed Healthcare Executive, Steelman suggests that creating "certainty in an uncertain world" in the digital therapeutic space could potentially open up doors for further use of these tools.

Mike Baldzicki, chief commercial officer, Premier Pharmacy Services, shared some takeaways from his presentation on the rare-ultra orphan disease market and its therapies at the 2023 PBMI Annual National Conference in Orlando.

At the 2023 PBMI Annual National Conference in Orlando, Ellen Feeney, director of Clinical Pharmacy Strategies at Highmark discusses how these new programs payers are using to control medication costs work, and shares how costs of the GLP-1s should be managed.

Ittai Dayan, M.D., M.P.H., co-founder and CEO of Rhino Health, is one of 12 winners in this year's Emerging Leaders in Healthcare feature. In this video interview, Dayan not only shares a good read for healthcare professionals, but also a turning point that changed his career trajectory and even a guilty pleasure of his — which involves finishing a scoop or more of his son's ice cream.

Ozempic (semaglutide) is second only to Humira (adalimumab) in sales, according to the IQVIA vice president of industry relations and a frequent speaker at conference. And for newly launched drugs, sales figures for Mounjaro (tirzepatide) are second only to what they were for Harvoni (ledipasvir and sofosbuvir), a hepatitis C antiviral, when the hepatitis C antivirals were new.

At this year's PBMI Annual National Conference in Orlando Ben Urick, principal health outcomes researcher at Prime Therapeutics, shared methods for medical cost savings through adherence improvement using integrated medical and pharmacy claims data.

Sampson Davis, M.D., an emergency medicine physician and best-selling author, says a tailored approach is needed.
Case and care management can help health plans cope with seven-figure cell and gene therapies, according to panelists at the Pharmacy Benefit Management Institute’s annual meeting.

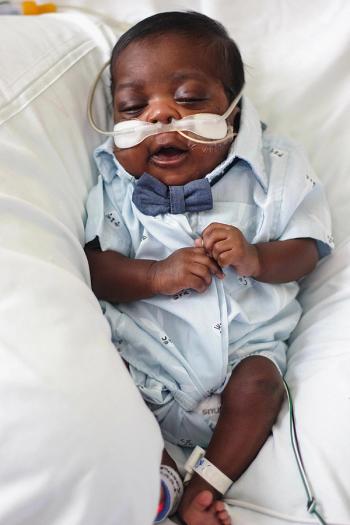
The mortality rate has dropped for Black premature infants, but they are still 1.4 times more likely to die than White premature infants, according to findings reported in JAMA Pediatrics today.

A special registration process could strike the right balance between increasing access to the drug used to treat opioid use disorder while limiting diversion and possible misuse, say an MHE editorial advisory board member and two co-authors.

Proponents hailed yesterday's announcement of the first 10 drugs subject to Medicare "maximum fair price" starting in 2026 as ushering in an era of lower drug prices and a political victory over pharma. Critics said it is price controls masquerading as negotiation and would smother innovation in one of the most innovative industries in the U.S.

Topping the list is Eliquis, which accounted for $16.48 billion in spending for the 3.7 million people in Medicare Part D who take the blood thinner.
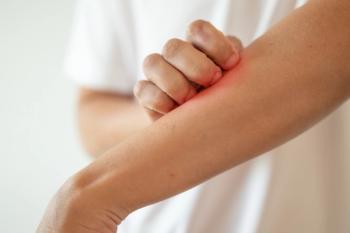
Chronic prurigo is a chronic itchiness can set in motion an itching -scratching cycle that results in scarring. A case series at a hospital in Zurich, Switzerland, shows Dupixent (dupilumab) injections are an effective treatment.
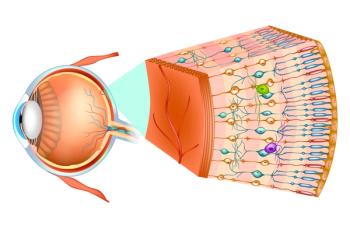
AXNOO7, an investigational agent for geographic atrophy, did not affect geographic atrophy lesions but showed positive effects of tests of visual acuity. A novel, neuroprotective mechanism of action may be why.
The manufacturer has reported seven causes of retinal vasculitis associated with Syfovre. Results of an open-label, extension study presented at the American Society of Retina Specialists meeting today show favorable results among those who crossed over from sham to active treatment.
The approval of an ophthalmic bevacizumab biosimilar might mean the end of low-cost, repackaged Avastin as an anti-VEGF treatment for retinal diseases, with the unintended consequence of adding $457 million to Medicare B spending.

In an interview with MHE, Parikh, a retina specialist in New York and director of healthcare delivery research for the Department of Ophthalmology at NYU Grossman School of Medicine, described the current situation of biosimilars in retinal medicine and the attitudes of retinal specialists about using the lower-cost alternatives.

Researchers used the American Academy of Ophthalmology's IRIS database to quantify the risk of endophthalmitis — inflammation of intraocular fluids — after injections with anti-VEGF drugs. They found that post-injection endophthalmitis occurs, on average, once every 3,500 injections.

Samsung Bioepsis' SB15 was comparable to Eylea (aflibercept) in a 56-week randomized clinical trial that included a rerandomization at 32 weeks. The rerandomization resulted in 111 study volunteers switching from Eylea to the biosimilar for the last part of the study.