
Papzimeos is the first and only FDA-approved treatment for adults with recurrent respiratory papillomatosis (RRP), with ongoing clinical studies showing symptom relief for up to two years.

Papzimeos is the first and only FDA-approved treatment for adults with recurrent respiratory papillomatosis (RRP), with ongoing clinical studies showing symptom relief for up to two years.

This final part of the three-part video series highlights Sharon Faust of Navitus reflecting on how personal challenges, including her husband’s accident, reshaped her perspective and empowered her to lead boldly in the PBM industry.

Those with hemophilia face heightened risks of depression and anxiety due to pain and joint issues, impacting their overall mental health and quality of life.

Children with HIV face a shortened life span, educational barriers and daily stigma, according to Amir Ardeshir, Ph.D., DVM, associate professor of microbiology and immunology at Tulane University.

In this video interview, Amanda Bogle, a healthcare attorney at Sheppard Mullin, explains the importance of compliance officers, documentation systems and leadership for pharmacists looking to adhere to the evolving regulatory landscape.

Veena Joy, the U.S. Lead for Strategic Partnerships, Allergy and Autoimmunity at Thermo Fisher Scientific, discusses the latest advancements in autoimmune disease research and explains why industry collaboration is essential to finding disease cures.

Postmenopausal women who sit less may have better heart health outcomes than those who sit for longer periods of time, according to a recent study published in Circulation.

Pharmacists and prescribers express ongoing concern about medication prior authorization struggles, but most are willing to adopt new technologies to ease the burden, according to a recent Surescripts survey.

HIV-fighting antibodies produced in infant macaques in a preclinical trial could be the future of HIV prevention in humans, according to Amir Ardeshir, Ph.D., DVM, Associate Professor of Microbiology and Immunology at Tulane University.

Here’s what you missed this week on Managed Healthcare Executive.
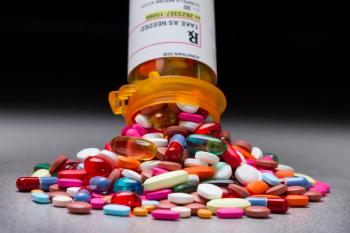
A recent study found major racial disparities in U.S. prescription drug use, with White patients using the most and Black patients often undertreated.

Access is the central tenet to the success of lenacapavir as a form of preexposure prophylaxis (PrEP), according to Jeremiah Johnson, the executive director of PrEP4All and Mitchell Warren, the executive director of the AIDS Vaccine and Advocacy Coalition (AVAC).

Teva's Ajovy gains FDA approval for pediatric migraine prevention, offering hope for children aged 6-17 with this debilitating condition.

Risks and benefits associated with mail-order pharmacies and third-party platforms, according to Amanda Bogle, a healthcare attorney at Sheppard Mullin.

Without guardrails, artificial intelligence can be abused, leading to overbilling and high volumes of appeals. But with the right strategy, AI can enhance — not compromise — the integrity of care and payment.

Medicare Advantage usage trends reveal slight declines in 2023, highlighting critical insights for policymakers amid rising healthcare costs and enrollment shifts.

Pharmacies and PBMs face growing regulatory and reporting demands around drug pricing and reimbursement, while misalignment between state rules and payer practices complicates the expanded clinical role of pharmacists.

Dove’s new line of body care products was developed to address symptoms often associated with menopause, such as skin dryness and body odor.

International audience surveys from last year’s menopause documentary, The (M) Factor, shed light on the ongoing need for increased menopause awareness for patients and providers alike.

Pavblu, an Eylea biosimilar, shows stable vision outcomes and minimal side effects in real-world use, promising a reliable treatment option for patients.

Veena Joy, U.S. Lead for Strategic Partnerships, Allergy and Autoimmunity at Thermo Fisher Scientific, discusses the latest in autoimmune disease research and explains why it’s a “good storm,” but not a perfect one.

Here’s what you missed this week on Managed Healthcare Executive.

Homecare Homebase revealed over 4 million patients are denied home health services annually, highlighting critical access issues and the impact of proposed Medicare cuts.

Small doses of the involuntary nervous system blocker, atropine, may offer a non-invasive treatment for symptomatic vitreous floaters, according to a recent small study.

The FDA has approved VIZZ, a once-daily, preservative-free eye drop that improves near vision in adults with presbyopia for up to 10 hours.

More than 60 companies pledged to improve healthcare technology during a White House “Make Health Tech Great Again” event hosted as part of the Centers for Medicare & Medicaid Services Health Tech Ecosystem initiative.

New findings reveal Syfovre may protect central vision in macular degeneration patients, delaying vision loss and enhancing treatment strategies.

New findings reveal that early treatment with Syfovre significantly improves vision preservation in geographic atrophy patients, according to Robert Goldberg, M.D., MBA, of the Bay Area Retina Associates.

CVS Health reports Q2 earnings decline due to litigation charges but boost adjusted EPS guidance, highlighting transformation and growth in healthcare services.

Although data breaches in the healthcare industry cost less than they did last year, healthcare is still the most targeted industry for cybercriminals for the fourteenth consecutive year.