
Those who participated in rehabilitation programs were less likely to die and had improved overall function, a recent study finds.

Those who participated in rehabilitation programs were less likely to die and had improved overall function, a recent study finds.

In COVID-19 news, FDA expanded EUA for Pfizer/BioNTech COVID-19 booster to children 5 to 11 years and cleared first at-home combo COVID-19, RSV and flu test, but declined an EUA for the antidepressant fluvoxamine to treat COVID-19. Regulators also approved Lilly’s novel diabetes drug and Dupixent eosinophilic esophagitis, modified Dsuvia REMS program and issued a CRL for bimekizumab for psoriasis.
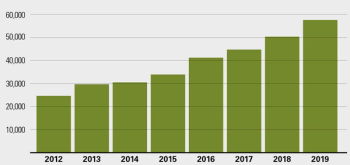
Rates dipped in the 1990s, but the opioid epidemic and the contaminated needles associated with it have caused an increase in new acute cases.

A recent HHS Office of Inspector General's report found that Medicare Advantage (MA) plans inappropriately deny prior authorization requests. With MA enrollment growing, scrutiny of MA plans and their utilization management strategies is also likely to grow, according to Alina Czekai, M.P.H., of Cohere Health. Czekai argues that artificial intelligence and machine learning can improve utilization management and prevent inappropriate denials.

Florida Blue and Blue Shield of California are among the insurers curating and offering free access to wellness apps for their members.

A list of biosimilar competitors to Humira that may come on the market next year.

Circle 2023 on your calendar. That's when as many as 11 biosimilars to Humira are headed for the U.S. market.

Kate Sommerfeld, president of the Social Determinants of Health Institute, spoke about efforts to address social determinants of health (SDOH) gaining traction, taking an interconnected approach and using data analytics to understand the value of the efforts to address SDOH.

There’s a long history to recognizing that social factors shape people’s health. But the social determinants of health are getting more attention than ever. Will that attention turn into action and lasting change?

Across the globe, new drugs — injections, topical and oral — are being developed to help patients with atopic dermatitis.

Research suggests that COVID-19 vaccination may serve a therapeutic effect, possibly reducing the risk of developing long COVID when administered to those currently infected.
Data suggest most patients have positive serology results a month following vaccination with either the Moderna or Pfizer vaccine.

In COVID-19 news, the FDA has approved Olumiant for new COVID-19 indication, but had limited Janssen’s COVID-19 vaccine. The agency has launched a new program for rare disease drug development and approved a new oral form of ALS therapy. Regulators have also extended the review time for both a new Pompe disease therapy and the sNDA for Myfembree for endometriosis. Additionally, Eisai has completed its submission of lecanemab for Alzheimer’s disease.

The partnership will allow international manufacturers to use the technologies for the potential development of COVID-19 vaccines, treatments and diagnostics to benefit people living in low- and middle-income countries.

Pirfenidone is used to treat patients with idiopathic pulmonary fibrosis, a progressive rare disease that make it difficult to breathe.

It is not clear whether gut inflammation has a causal role in myeloproliferative neoplasms, or the other way around.

Research and discussion about the high cancer rates among Black Americans used to be dominated by genetics and the search for biological differences. Now attention has shifted to the social determinants of health.
Efforts to improve education on biosimilars are a start, but payer policies must catch up to ensure real change.

Price-transparency rule allows insurers to cut MLR payments by using incentives to encourage consumers to choose low-cost, high-value providers.
Multiple patents on original biologics delay when biosimilars get on the market in the US. University of Denver Sturm College of Law legal experts argue in a preprint that changes in how the original patents are written could reduce the number of duplicative patents and legal gamesmanship.
A Cleveland Clinic researcher anticipates that the FDA’s definition of strength of formulation could impair acceptance of Boehringer Ingelheim’s biosimilar to Humira (adalimumab), even though the FDA has approved the biosimilar as “interchangeable” to Humira.
Findings from phase 2 trials reported in Journal of Clinical Oncology seed hopes that winnowing out certain types of T cells from peripheral blood stem-cell transplants will make chronic graft-versus-host disease less common. Randomized trials are underway to test the proposition.

High engagement was linked with high weight loss, the analysis shows.
The administration of adult stem cells increased survival in patients with both acute and chronic graft-versus-host disease in a compassionate use program.
Safe and effective long-term treatment is important for stable disease control in atopic dermatitis.

Healthcare insurance in the U.S. can be spotty, even with expansion of coverage under the Affordable Care Act. One of the remaining problem areas is coverage for people who are leaving prison or jail.

The FDA has approved a rare disease therapy and an additional indication for Enhertu, and grants priority review for Imfinzi in biliary tract cancer. Regulators have issued complete response letters for three therapies. The agency also has scheduled an advisory committee for Nuplazid in Alzheimer’s psychosis.

Beauty may be in the eye of the beholder, but drug price trends are in the way you crunch the numbers.
Bortezomib is part of the company’s KabiConnect program, a recent expansion of its KabiCare patient support program

Politics and policies were among the main topics at the meeting in Las Vegas. But the 6,000 attendees also heard about biosimilars, the gross-to-net price gap and the ripple effects of the CMS’ Aduhelm coverage decision.