Researchers say kappa free light chain tests would be less costly and produce results sooner than the oligoclonal band tests.

Researchers say kappa free light chain tests would be less costly and produce results sooner than the oligoclonal band tests.

CMS’s final decision is that monoclonal antibodies to treat Alzheimer’s disease may only be covered in a randomized controlled trial conducted under an investigational new drug application.

A new kind of specialist is needed at academic medical centers to cope with public health emergencies such as the COVID-19 pandemic: physician-public health practitioners.

Enrollment in Affordable Care Act marketplace plans has reached record levels, and the Biden administration is using those numbers to push for continuation of enhanced subsidies that have lowered premiums for the plans.

Marcus Snow, MD, , chair of the American College of Rheumatology’s Committee on Rheumatologic Care, discusses how additional clinical data and interchangeability designations could help build momentum for use of Humira (adalimumab) biosimilars when they start to arrive in 2023.
In a pair of recent studies, investigators evaluated biosimilar forms of Humira (adalimumab) in prefilled syringes, prefilled pens, and autoinjector devices.

The Access to Prescription Digital Therapeutics Act, introduced in March, has the backing of the Academy of Managed Care Pharmacy.
A therapy that rapidly demonstrates improvements in clinical outcomes may have a positive impact on medication adherence.

Briana Contreras, editor of Managed Healthcare Executive spoke with Emad Rizk, M.D., chairman, president, and CEO of Cotiviti in this week's episode of Tuning In to the C-Suite. In the discussion, they talked about the issues of risk in healthcare and how they have led to inequalities in care. They also addressed how adopting risk assessment tools that expand access to data around social determinants of health can help reduce bias and improve care.

Using an aggressive utilization management approach, Providence St. Joseph Health directed physicians toward the use of biosimilars immediately after they came to market, saving nearly $27 million over two years in the process, according to Sophia Z. Humphreys, Pharm.D., M.H.A., the healthcare system’s director of system pharmacy clinical Services.

About 3,000 people attended the Academy of Managed Care Pharmacy's annual meeting in Chicago last week.

HIV PrEP uptake was highest for White and Black males and lowest for Hispanic males. Meanwhile, Black females had the highest uptake and twice the rate of PrEP uptake of White females.

The proposed funds include $165 million more for the Ryan White HIV/AIDS Program, $47 million more for HIV and hepatitis prevention activities at the Indian Health Service, and $115 million for CDC HIV prevention programs.
Long-acting injectable holds advantages over daily pills for pre-exposure prophylaxis against HIV, says ViiV executive.

The FDA authorizes second COVID-19 booster and approves a higher does of Ozempic, Cabenuva for adolescents with HIV, a therapy for rare seizer disorder, and an oral testosterone replacement. The agency issues a complete response letter for a therapy for anemia related to chronic kidney disease, and an advisor committee votes down a drug for ALS.
Camcevi is the first ready-to-inject sterile formulation of leuprolide mesylate for subcutaneous injection that comes in a pre-filled syringe with no mixing required.

Pear Therapeutics’ Yuri Maricich says the company hasn’t “put a stake in the ground” on the question but says standardizations is important.

A COVID-19 risk score helped steer outreach efforts to people who weren’t getting vaccinated by the pharmacy chain earlier in the pandemic.

VBI Vaccines’s PreHevbrio [Hepatitis B Vaccine (Recombinant)], the only approved 3-antigen hepatitis B vaccine for adults, is now commercially available in the U.S. after approval by the FDA late last year.
The overall number of deaths involving alcohol spiked 25.5% to 99,017 between 2019 and 2020.

The prevalence of nonalcoholic fatty liver disease (NAFLD) is significantly higher in men (33%) than women (20%).

Zahra Mahmoudjafari, PharmD, BCOP, clinical pharmacy manager of Hematology/BMT/Cellular Therapeutics at University of Kansas Health System discussed CAR T-Cell therapies in managed care at the annual AMCP 2022 conference in Chicago.
Katie Lockhart, MA, manager of Magellan Health, addresses her AMCP 2022 conference session on the financial impact of specialty drugs and cost-impact models.

Soumya Vishwanath, PharmD, senior manager of Formulary Strategy at Magellan Rx Management, talked about digital therapeutics in the behavioral health space and formulary strategy space during the annual AMCP 2022 conference in Chicago.
“Popping a pill” makes cancer treatment more convenient, but research presented at the AMCP annual meetings shows that almost half of cancer patients on oral therapies are nonadherent. Oral administration typically moves a drug to the pharmacy benefit, which can mean high out-of-pocket costs and, in turn, nonadherence.

Over 130 digital therapeutics for behavioral health are in the pipeline, but many payers are still sussing out how best to evaluate them and perhaps provide coverage, according to panelists at AMCP 2022. And for all of their advantages, digital therapeutics have some notable limitations and drawbacks.

Michael Ciarametaro, MBA, National Pharmaceutical Council National discusses gene therapy and the Medicaid Best Price Rule with MHE during the annual AMCP conference in Chicago.
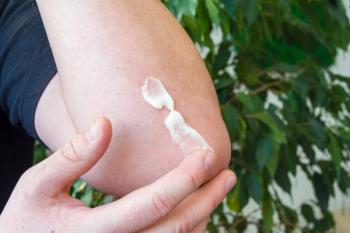
Dupixent (dupilumab) ushered in a new era and a growing number of treatment options has brought awareness to the condition. But the panelists also discussed problems with step therapy, delays and denials of treatment and the lack of a “gold standard” test for diagnosis.

Patients who live in rural areas incur a variety of costs attributed to physically getting into the office to meet with their doctor when the need arises. When those patients require more frequent visits for chronic disease management, the cost of transportation, missed work, out-of-pocket copays, and any number of other costs can become a substantial barrier to care.

The healthcare sector added 63,500 jobs in February alone – a kind of growth in healthcare employment hasn’t seen since pre-pandemic days. In addition to the healthcare industry alone, the senior care sector reported its third employment gain in a row with a total year-to-date increase of 9,000 jobs.