
A new Tufts study found that Medicare price negotiations on biologics with imminent biosimilars could lead to near-term savings, but greater savings are likely from biosimilar competition.

A new Tufts study found that Medicare price negotiations on biologics with imminent biosimilars could lead to near-term savings, but greater savings are likely from biosimilar competition.

High consumption of ultra-processed foods elevates the risk of early-onset colorectal cancer, highlighting the need for improved dietary quality for prevention.
Results from a recent ianalumab treatment trial signal a potential for longer-lasting disease control for patients with primary immune thrombocytopenia.
New study shows catheter ablation significantly was associated with a 40% drop in major cardiovascular events and a 70% decline in all-cause mortality for patients with both atrial fibrillation and obstructive sleep apnea.

Sanofi introduces Qfitlia earlier this year, a groundbreaking hemophilia treatment, offering flexible dosing and support for patients transitioning from existing therapies.

Jeff Schaffnit discusses Sanofi's advancements in BTK inhibitors and promising results for hemophilia A therapies at ASH 2025, enhancing treatment options.
Melanoma survival rates have doubled since 2009. New treatments, including oncolytic viruses, fecal transplants, and engineered antibodies, have shown promising results in clinical trials.

Clinicians are moving toward managing mild (Grade 1) cytokine release syndrome on an outpatient basis.

The CAR T Vision Steering Committee members wish to double the number of eligible patients receiving chimeric antigen receptor (CAR) T-cell therapy by 2030 through disease awareness and sustainable financing options, according to George Eastwood, Executive Director of the Emily Whitehead Foundation and recent CAR T Vision Steering Committee member.

New drug approvals in dermatology in 2025 included a gene therapy for rare skin disorders and targeted treatments for two conditions.

Flipped dosing of Yervoy and Opdivo produced a 49% response rate compared with 37% for the standard dose of the combination and extended survival to 42 months from 14 months in a Swedish study.

As the number of patients with multiple myeloma and lymphoma treated with CAR-T therapy increases, health systems are shifting post-infusion care from inpatient to outpatient settings. Research presented at the annual meeting of the American Society of Hematology shows the change slices costs by $19,180.
Tranexamic acid given during noncardiac surgery reduced blood transfusions by 2.4% without increasing blood clots, potentially saving millions of transfusion units annually nationwide.

A phase 3 trial showed that Jaypirca improved progression-free survival in newly diagnosed patients with chronic lymphocytic leukemia and small lymphocytic lymphoma compared with standard chemotherapy/rituximab regimen.

Dermatologists explore chronic itch advancements, revealing new treatments and insights into its complex biology, lifestyle impacts and future care strategies.

Chimeric antigen receptor (CAR) T-cell therapy can cure certain types of blood cancer, but cost and a general lack of awareness around the therapy are barriers to care, according to George Eastwood, Executive Director of the Emily Whitehead Foundation.

In this interview, George Eastwood, Executive Director of the Emily Whitehead Foundation and CAR T Vision steering committee member discusses barriers and strategies for expanding access to CAR T-cell therapy.
Early results from the LINKER-MM4 trial show high response rates for linvoseltamab, offering new hope for newly diagnosed patients.

Early results from the LINKER-MM4 trial reveal promising response rates for linvoseltamab in newly diagnosed multiple myeloma patients, with manageable side effects.

Jaypirca demonstrated non-inferior response rates compared with Imbruvica in patients with chronic lymphocytic leukemia and small lymphocytic lymphoma, with fewer cardiovascular events and dose reductions.

Here’s what you missed this week on Managed Healthcare Executive.
Venclexta-azacitidine combination offered better responses with less hospitalization and lower symptom burden compared with standard chemotherapy in patients with acute myeloid leukemia who are fit for transplant.

The chief medical officer of Genetix Biotherapeutics, formerly known as Bluebird bio, discusses timelines and operational challenges of the gene therapies Zynteglo for beta thalassemia and Lyfgenia for sickle cell disease.

About one-third of families with a child undergoing treatment for acute lymphoblastic leukemia experienced catastrophic income loss during therapy, with many developing new material hardships over the two-year course, underscoring the need for ongoing financial screening and support.
IV iron infusions were found to be safe for anemic patients hospitalized with acute bacterial infections and were associated with improved survival and higher hemoglobin levels, challenging long-held concerns that iron worsens infections.

The patient died from vent-occlusive liver disease, a well-characterized side effect of the busulfan, the myeloablative chemotherapy used to prepare the bone marrow for engraftment with the Casgevy-modified blood stem cells.
Adults with sickle cell disease face longer emergency department wait times for pain treatment than children, with women experiencing more delays than men.


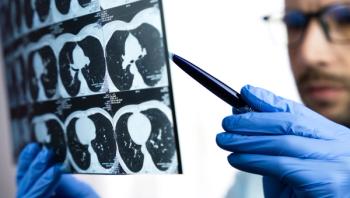
The AI-powered imaging technology quantifies the extent of fibrosis on CT scans and also measures other features, such as ground-glass opacification.