
Current drug shortages based on FDA data, prepared by Formulary Watch.

The therapy for HIV-related pneumonia was exposed to cold weather during shipment.

The therapy can now be used for post-exposure prevention in people at high risk for progression to severe disease.
The PDUFA date has been set for December 4, 2021; FDA has designated this as a Priority Review.

The PDUFA date for this indication is December 4, 2021.

A single application targets the infection without entering the circulatory system.
The FDA is expected to make a decision on this indication by December 1, 2021.
The therapy had received accelerated approval in 2011 but a phase 3 confirmatory trial found it did not meet the end point of progression-free survival.

The sickle cell disease therapy will now be on the Preferred Drug List.
This is the first new approval for lupus in a decade.

Pharmacies will need to obtain pre-dispense authorization for clozapine.
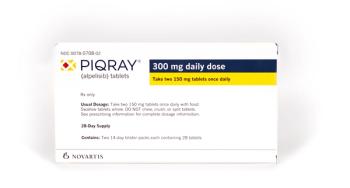
Some fatal cases of ketoacidosis have occurred, and high-risk patients should be monitored when starting treatment.

The FDA is requesting an additional study to demonstrate tenapanor lowers serum phosphorus.

Drugs that provide only incremental benefit may not be included on clinical pathways.
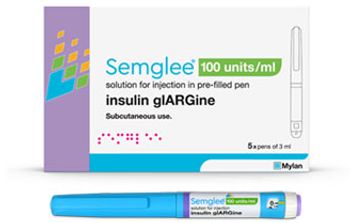
The approval allows for substitution at the pharmacy counter for Lantus, its reference product.
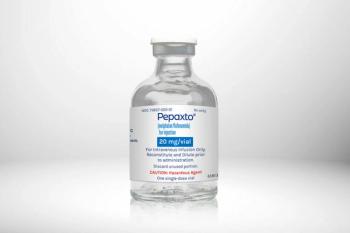
The agency has stopped enrollment in all ongoing trials.

The findings were similar in people with or without diabetes.

Competition among branded products did not substantially curb annual spending increases.
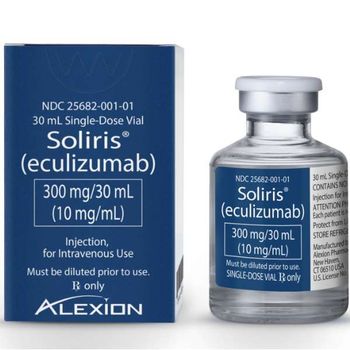
A substantial discount would be needed to meet cost-effectiveness thresholds.

The FDA would like to see additional data on clinical benefit for patients with anal cancer.
Angiotensin receptor blockers may be less likely to cause side effects than ACE inhibitors.

Current drug shortages based on FDA data, prepared by Formulary Watch.

Keytruda gets a full approval in endometrial cancer; while Xeljanz will miss another PDUFDA date.

When Medicare D plans do provide coverage for first generics, they are often placed on non-generic tiers.

The June 30 approval meets an unmet need in this cancer.