Reblozyl was being reviewed to treat anemia in adults with non-transfusion dependent beta thalassemia, an inherited blood disorder. BMS indicated it couldn’t appropriately address FDA’s questions about risk-benefit.

Reblozyl was being reviewed to treat anemia in adults with non-transfusion dependent beta thalassemia, an inherited blood disorder. BMS indicated it couldn’t appropriately address FDA’s questions about risk-benefit.

New launches this week include: new strength of Alimta, a generic of Esbriet, a generic of Toradol, and a generic of Florinef.
The FDA has extended review time to allow for review of additional analyses of data from the company’s clinical studies. The new PDUFA date is Sept. 29, 2022.
The label now includes a new section about the risk of substituting an oral azacitidine product, Onureg, for the injection therapy.

The FDA is asking for additional data to support effectiveness of pegzilarginase to treat a rare metabolic disease. If approved, pegzilarginase would be the first treatment for ARG1 deficiency.

More than 5,700 trials worldwide are investigating PD-1/PD-L1 inhibitors, and new trials of these therapies were of combination regimens.
Byooviz, the first FDA approved ophthalmology biosimilar for macular degeneration, will be priced 40% lower than the reference product. It will be commercially available on July 1, 2022.

The regulatory agency indicated there is an issue surrounding the protocol design.

If approved, Dupixent would be the first and only medicine in the United States specifically indicated to treat prurigo nodularis, or persistent itch with thick skin lesions. The PDUFA target action date for the FDA decision is Sept. 30, 2022.

The FDA indicated the risk of death outweighs the benefits of Ukoniq, which was approved in February 2021 to treat specific lymphomas.

In high-deductible plans, patients had the highest out-of-pockets costs and abandoned and discontinued therapy at greater rates than other benefit designs.

Evrysdi is now approved to treat spinal muscular atrophy in children and adults of all ages.

Opdivo-based treatments are now approved for five indications in upper gastroesophageal cancers.

The bill would allow the Federal Trade Commission to impose penalties on PBMs of up to $1 million for unfair and deceptive practices.
Kymriah is now approved in three indications and is the only CAR-T cell therapy approved in both adult and pediatric settings.

The Access to Oncology Medicines Coalition brings together pharma companies and other organizations to help countries develop the capacity and access to essential cancer medicines.

This is the fifth biosimilar of Neulasta and the third biosimilar from Amneal to receive FDA approval.

The FDA has assigned a Prescription Drug User Fee Act target action date of Nov. 30, 2022. Regulators indicated they plan to hold an advisory committee meeting to discuss the application.
Tibsovo is the first therapy targeting cancer metabolism for patients with newly diagnosed IDH1-mutated acute myeloid leukemia.
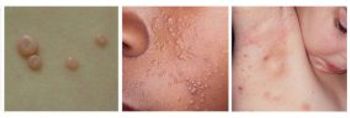
Deficiencies at the contract manufacturing company are, for the second time, holding up approval of VP-102 to treat molluscum contagiosum, a mild skin infection.
If approved, etranacogene dezaparvovec would be the first gene therapy for patients with hemophilia B. An FDA decision is expected in November 2022.

Molly Beinfeld, senior research lead, evidence synthesis at the Institute for Clinical and Economic Review, talks about the organization’s research assessing payer coverage policies of prescription drugs.

The impacted lot failed a dissolution test, meaning it was taking longer to dissolve once ingested. This can result in less anagrelide available in the body, possibly leading to clotting or bleeding events such as a heart attack or stroke.

Vtama is approved to treat adults with mild, moderate, and severe psoriasis. It is expected to be available in the first week of June 2022.

Tyvaso DPI is a dry inhalation powder and new inhalation device to treat patients with pulmonary arterial hypertension, a life-threatening disease. It is expected to be available in June 2022.

Doug Nemecek, M.D., chief medical officer, behavioral health at Evernorth, talks about the mental health issues teenagers are facing.