Ibtrozi had a 90% response rate in treatment naïve adults with locally advanced or metastatic ROS1-positive non-small cell lung cancer, according to the results of a news release.

Ibtrozi had a 90% response rate in treatment naïve adults with locally advanced or metastatic ROS1-positive non-small cell lung cancer, according to the results of a news release.

Enflonsia is the first FDA-approved respiratory syncytial virus (RSV) preventative for infants, regardless of weight, according to the news release.

A survey by PSG has found that the pressure to control drug costs is leading some payers to look into unbundling pharmacy benefits as a way for better control.

Switching to Humira biosimilars saved Navitus clients more than $315 million in upfront costs in 2024 and resulted in a 60% reduction in net costs per claim.

The pharmacy benefit manager (PBM) industry is under pressure to shift to models that avoid conflicts of interest and misaligned incentives.
The approval is based on results from the phase 3 ARANOTE trial, which showed Nubeqa, when combined with androgen deprivation therapy (ADT), reduced the risk of radiographic progression or death by 46% compared to ADT with placebo.

State efforts are aiming to bring more disclosure and transparency to PBM practices, mandating certain pharmacy reimbursement levels, requiring 100% pass-through rebates, and prohibiting spread pricing.
Pharmacy costs and outpatient facility care generate 69% of the cost increase for 2025, according to Milliman’s recent Medical Index.

Jason R. Smith, Pharm.D., appointed as chief pharmacy officer in February 2025 for the University of Rochester Medical Center, talks about building a stronger workforce, managing drug shortages and keeping up with changes in regulations because of the new administration.
Nucula’s new indication is as an add-on therapy for patients with COPD who also have an elevated blood eosinophil count.
Susvimo is also approved to treat diabetic macular edema and age-related macular degeneration, and the medication, ranibizumab, is delivered through an ocular implant that is refilled every nine months.

Health plan sponsors are also expected to see a reduction in the net cost per prescription of GLP-1 medications to treat obesity.
Bayer’s Jivi is a factor VIII replacement therapy that has been engineered with a compound that allows for less frequent dosing. It is now indicated for patients 7 years of age and older.

Zynyz is now FDA-approved as the first and only approved first line treatment for advanced squamous cell carcinoma of the anal canal.

A new law in Iowa, if signed by the governor, will mandate 100% pass-through of rebates, increased financial transparency, and a minimal payment for pharmacies. Critics say it will be the most costly mandate in the state’s history.

It could improve access to community pharmacies and lower prices. Or it will limit access to critical drugs and impact payers’ ability to contract for a broad range of services. Industry leaders are unsure about the impact of Arkansas’ law banning PBMs from owning pharmacies.
Express Scripts, Cigna, UnitedHealthcare, CVS Caremark and Optum Rx are among the payers that have added Yesintek to their formularies.
If approved, Wegovy would be the first oral GLP-1 drug to treat obesity.

Adult and pediatric generalized myasthenia gravis patients have a new, longer acting option for treatment called Imaavy.

Effective July 1, 2025, CVS Caremark will place Wegovy injection 2.4 mg as preferred on its commercial template formularies.

Insurance hurdles and the complexities of prior authorization create barriers to care, according to oncologists in a new survey by Sermo.
Novo Nordisk has teamed up with Hims & Hers Health, LifeMD and Ro for cash-paying patients to access Wegovy injection 2.4 mg through its NovoCare Pharmacy.

Giant Eagle also helped EmpiRx Health create a national pharmacy care network that was purpose-built for pharmacy and grocery chains.

Nationwide, 58% of Medicare beneficiaries living in rural areas are enrolled in stand-alone prescription drug plans in 2025.

The complete response letter did not identify any issues with the safety or efficacy of Eylea HD, Regeneron officials said.
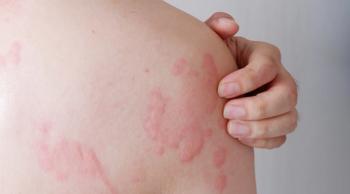
Chronic spontaneous urticaria is chronic inflammatory skin disease driven in part by type 2 inflammation, which causes sudden and debilitating hives and recurring itch.