
CSF-1 is a low-dose pilocarpine to treat patients with age-related blurry vision. The FDA has assigned a PDUFA goal date of Oct. 22, 2023

CSF-1 is a low-dose pilocarpine to treat patients with age-related blurry vision. The FDA has assigned a PDUFA goal date of Oct. 22, 2023
Alnylam is seeking approval for Onpattro’s use in cardiomyopathy related to transthyretin-mediated (ATTR) amyloidosis. The FDA set an action date of Oct. 8, 2023.

Patients who have had liver problems in the past may be at risk of liver damage from Sprycel, which is used to treat patients with chronic myeloid leukemia.

The new once-daily formulation of Austedo is used to treat adults with tardive dyskinesia and chorea associated with Huntington’s disease.
If approved, RSVpreF would be the first vaccine for administration to pregnant women to help protect against RSV disease in infants. The FDA has set an action date for August 2023.
Filspari will be available for a wholesale acquisition cost of $9,900 for a 30-day supply, and administrated through a REMS program because of the risk of liver abnormalities.

Apellis’ Syfovre will have a list price of $2,190 per vial, and Medicare is expected to be the dominant payer.

The U.S. public health emergency response to COVID-19 ends May 11, 2023, and the transition to more traditional healthcare coverage will begin later this year.

The review has been extended by three months. The new Prescription Drug User Fee Act (PDUFA) date is May 22, 2023.
At a placeholder price of $19,000, resmetirom would produce cost savings, says the drug evaluation group. Obeticholic acid’s price would need to be slashed to meet a commonly used cost-effectiveness threshold.

Lamzeda has been approved to treat patients with alpha-mannosidosis, a rare genetic condition. It will be available in the first half of this year.

Cuban, of "Shark Tank' fame and owner of the Dallas Mavericks, called drugs prices ‘insane’ and used some profanity when talking pharmacy benefit managers at the annual meeting of the Association for Accessible Medicines yesterday. Cuban has co-founded an online pharmacy that he says will make drug prices lower and transparent.

Navitus will be accessing the Humira biosimilar Amjevita through the lower wholesale acquisition cost option.

The Prescription Drug User Fee Act action date is June 5, 2023. Prevymis is already approved to prevent CMV infection in adults after an allogeneic hematopoietic stem cell transplant.
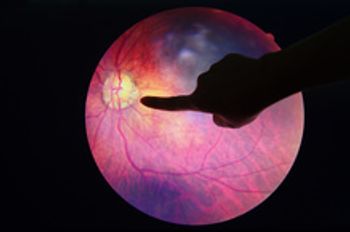
Avacincaptad pegol has the potential to slow the progression of geographic atrophy in age-related macular degeneration. The PDUFA goal date is Aug. 19, 2023.
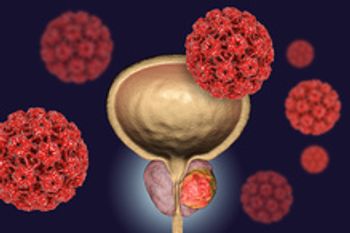
Phase 3 data showed that the combination resulted in a 37% reduction in risk of disease progression or death in men with advanced prostate cancer.
Senators want more insight into PBM practices and the role they play in the healthcare ecosystem.

Abarca's Nicole (Gupta) Bulochnik, Pharm.D., talks about how personalized medicine could be integrated into value-based care to accelerate uptake.

Javier Gonzalez, Pharm.D., at Abarca Health, talks about how value-based pricing arrangements can align incentives among stakeholders for specialty disease management.

The branded therapies were removed in favor of generics effective April 2023. CVS Caremark also added 13 generics to its Performance Drug List.

The hearing will discuss the role of PBMs in healthcare and the Pharmacy Benefit Manager Transparency Act, legislation that has been submitted by Sens. Maria Cantwell and Chuck Grassley.

No list price was provided for Rebyota but Ferring is offering copay and patient assistance programs.

The FDA indicated that Soligenix’s application for HyBryte, a novel photodynamic therapy, was incomplete.

Hospira/Pfizer’s potassium phosphates product alone may produce daily aluminum exposures of up to twice of the FDA-recommended limit for children.

If approved, Linzess would be the first and only FDA-approved prescription therapy for functional constipation in children 6 to 17 years of age. The PDUFA date is June 14th, 2023.

Chris Blackley, co-founder and chief executive officer of Prescryptive Health, discusses the laws that states that are implementing to restrict PBM practices and the impact on patients and employers.