
Aponvie is the first intravenous version of aprepitant adults to prevent postoperative nausea and vomiting.

Aponvie is the first intravenous version of aprepitant adults to prevent postoperative nausea and vomiting.
Iheezo, approved in September 2022, is an ocular surface anesthesia used during cataract surgeries.

Zavzpret is the first calcitonin gene-related peptide (CGRP) receptor antagonist nasal spray approved for patients with acute migraine.

Radicava ORS will be available with a national prior authorization process in place.

Other news includes the launches of the first generic of an ulcer drug, the antipsychotic lurasidone, an extended-release version of topiramate for migraine, and additional strengths of the cancer drug lenalidomide. Additionally, Glenmark will distribute Cediprof's generic Adderall, and the FDA has accepted Amneal’s application for a generic naloxone nasal spray.

Payers are looking to pharmaceutical companies for innovative partnerships that provide patient access, positive clinical outcomes and reduced costs in specialty disease areas, AmerisourceBergen finds.

If approved, Jardiance would be the first SGLT2 inhibitor indicated for this population. A decision is expected in the second quarter of 2023.

Voxzogo is already available to treat patients older than five years with a genetic cause of dwarfism. The FDA has set a PDUFA of Oct. 21, 2023, for children younger than 5.
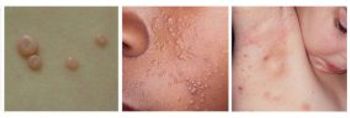
If approved, berdazimer could be the first prescription product treat molluscum, a common skin infection caused by poxvirus. The Prescription Drug User Fee Act goal date is Jan. 5, 2024.

With a Prescription Drug User Fee Act action date of Dec. 22, 2023, eplontersen is an antisense medicine to treat patients living with hereditary transthyretin-mediated amyloid polyneuropathy, which results in nerve damage.

The agency has set a new PDUFA target action date of June 30, 2023, for Roctavian. If approved, it would be the first one-time gene therapy for hemophilia. A.

The majority of consumers surveyed by Let My Doctor Decide support reforms to improve access and affordability.
The FDA plans to hold an advisory committee meeting to discuss the Alzheimer’s therapy. The agency has assigned Prescription Drug User Fee Act target action of July 6, 2023.
Shigella cause an estimated 450,000 infections in the United States each year and an estimated $93 million in direct medical costs.
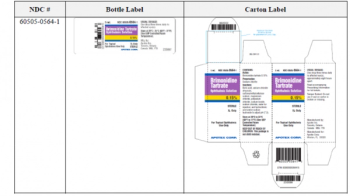
Brimonidine tartrate ophthalmic solution is used to reduce elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
Niraparib and abiraterone acetate plus prednisone has potential to address unmet need for patients with BRCA-positive metastatic castration-resistant prostate cancer.

If approved, ADX-2191 could be the first marketed drug specifically for patients with primary vitreoretinal lymphoma. The FDA assigned a Prescription Drug User Fee Act date of June 21, 2023.

Leqembi would need a 66% to 19% discount from its wholesale acquisition cost of $26,500 a year to fall within commonly used cost-effectiveness thresholds.

The price cuts apply to Lilly’s nonbranded insulin, Humalog, Humulin and its Lantus biosimilar.

Kevzara is the first biologic to treat patients with polymyalgia rheumatica (PMR). It is also approved to treat rheumatoid arthritis.
If authorized, Pfizer’s RSV vaccine would be used to prevent respiratory disease caused by RSV in adults 60 years of age and older. An FDA decision is expected by May 2023.

The companies have also submitted an EUA for a fourth dose of the bivalent COVID-19 vaccine in children 6 months through 4 years of age.

Skyclarys will be available in the second quarter of 2023 to treat patients with Friedreich’s ataxia, an ultra-rare, inherited neurodegenerative disorder. The wholesale acquisition cost is $370,000.

BMS is seeking an indication for Opdivo as monotherapy to treat patients with stage IIB or IIC melanoma after surgery. The FDA has assigned a Prescription Drug User Fee Act date of Oct. 13, 2023.

Investigators found an association between ICER’s value assessments and how U.S. commercial health plans cover specialty drugs.

The ads focus on how PBMs promote competition in the prescription drug marketplace and provide choice for employers.