Most of the Humira biosimilars are available with two pricing options. Of the larger PBMs, only Optum Rx so far has weighed in on a coverage decision.

Most of the Humira biosimilars are available with two pricing options. Of the larger PBMs, only Optum Rx so far has weighed in on a coverage decision.

Similar to Amgen’s Amjevita, Hulio will be offered at a list price of 5% below and 85% below the current Humira list price.
The increase is expected to be somewhat offset by the impact of biosimilars coming to market and more care being provided in outpatient settings.

How the insurance industry denies claims; consumers confused by complexity and red tape; and how the ALS drug Relyvrio came to be.

Recent study finds that Medicaid coverage for some of the first gene and cell therapies was at times delayed and not consistent with federal requirements.
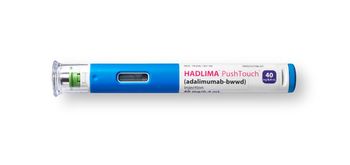
A carton of Hadlima including two pre-filled pens or two pre-filled syringes is available at a list price of $1,038.
In the revised guidance, CMS clarified aspects of the negotiation process and outlined additional opportunities for engagement during the process. CMS will publish by Sept. 1, 2023, the list of the first 10 drugs for price negotiation.

Biosimilars have the potential to lower costs, but the adoption has been slower than expected, a recent study finds.
BioMarin is offering an outcomes-based warranty that will reimburse payers up to 100% of the cost if a patient does not respond to Roctavian.
Longer-acting growth hormone reduces frequency of injections. It will be available in August.

In two small studies, patients were able to be free from insulin injections for a year or more.

Sarah Emond will continue with ICER’s efforts to assess drug cost-effectiveness and health plan access policies that began with founder Steve Pearson, M.D., who will transition to an advisor role in 2024.

The National Alliance of Healthcare Purchaser Coalitions has issued a playbook to help companies navigate what they see as a flawed PBM contracting model and lack of competition.
At issue is an ongoing review of inspection results from a third-party filling company. No issues were cited in the FDA’s complete response letter about clinical efficacy or safety.

It will be commercially available in the third quarter of 2023 and have a list price of $6,050 per vial.
Pfizer’s biologics license application (BLA) for the gene therapy fidanacogene elaparvovec has been assigned a PDUFA date in the second quarter of 2024.
Blincyto is immuno-oncology therapy that targets CD19 surface antigens on B cells to treat patients with acute lymphoblastic leukemia. Its current wholesale acquisition cost price is $4,900.15 per vial.

Now with the name Litfulo, ritlecitinib is the first oral treatment for adolescents with alopecia. It has an annual list price of $49,000.

Elevidys is a one-time therapy that delivers to muscle a gene that codes for a shortened, functional form of dystrophin, which is mutated in Duchenne muscular dystrophy.

Optum Rx will include the three biosimilars on its standard formulary at parity with Humira.
The company plans to discontinue further research of obeticholic acid to treat nonalcoholic steatohepatitis (NASH) after the FDA asked for long-term outcomes data.

Regulators indicated there was a lack of evidence of effectiveness and lack of clinical trials done to support the application.

Vyvgart Hytrulo’s under-the-skin administration means it can be given in 30 to 90 seconds, compared with one hour for the intravenous product. It will be priced at parity to Vyvgart, which has a net price of $225,000 annually.

Jardiance and Synjardy are the first SGLT2 inhibitors approved for children with type 2 diabetes.

Employers and plans need to prioritize clinical efficacy over drug price, look for price reductions — and not rebates — on biosimilars, and advocate for a public option to cover gene therapies. These are just a few of ideas that consultant Alex Jung put forward at a meeting of the Midwest Business Group on Health.

Imetelstat targets telomerase to inhibit uncontrolled proliferation of malignant stem and progenitor cells in myeloid hematologic malignancies.