
Following similar agreements, the settlement allows Fresenius Kabi and Formycon to launch their ustekinumab biosimilar no later than April 15, 2025.

Following similar agreements, the settlement allows Fresenius Kabi and Formycon to launch their ustekinumab biosimilar no later than April 15, 2025.
Biosimilars that are interchangeable with Humira could provide more competition on price, a new analysis suggests.
Payers’ requirements for the use of Camyzos in patients with obstructive hypertrophic cardiomyopathy are generally consistent with the registration study, but they also imposed restrictions beyond the FDA indication or ICER recommendation.
Talvey is a first-in-class bispecific antibody to treat adult patients with relapsed or refractory multiple myeloma. It will available in a few weeks and have a price of $45,000 per month.

Avasopasem was under review to treat radiotherapy-induced severe oral mucositis in patients with head and neck cancer. The FDA has indicted that an additional trial would be needed to show safety and effectiveness of avasopasem.

Current treatments urea cycle disorders, which causes ammonia to build up in the blood, have a bitter taste and smell. Pheburane was developed with a special coating that masks the taste of sodium phenylbutyrate. It has a list price of $4,375 per bottle.

Capital Blue Cross uses algorithms to help find drug manufacturer coupon programs. It has saved $4.5 million in the first quarter of 2023.

Almost half of adults surveyed by KFF would be interested in taking a weight-loss drug, including many who say they only want to lose a little weight.

This abstract at the American Society of Clinical Oncology (ASCO 2023) looked at the growing utilization of trastuzumab biosimilars over time in India.
The FDA has assigned a March 8, 2024, action date for GA Depot, a once-monthly injection to treat patients with relapsing forms of multiple sclerosis.

Izervay, which is expected to be available within the two to four weeks, will have a list price of $2,100 per vial.

Zurzuvae is a rapid-acting neuroactive steroid that is expected to be available in the fourth quarter of 2023.

The specialty pharmacy business is a driver of Cigna’s growth, accounting for 40% of the Evernorth’s revenue.

Researchers say capping insulin costs at $35 a month has the potential to lower events and costs related to diabetes, such as amputations, vision loss, and heart attacks.

CVS’s health services segment includes CVS Caremark, but the company would not break out the revenue the PBM business only.

Trial results showed that the combination of Lonsurf and bevacizumab provided improvements in overall survival.


The Association for Accessible Medicines (AAM) published a white paper on the impact that ongoing drug shortages have had on the generic and biosimilar industries, as well as possible solutions stakeholders can put into action.

Breyna is indicated for patients with asthma six years of age and as maintenance therapy for people with COPD.

Jemperli is the first immuno-oncology treatment approved in the frontline setting for patients with advanced or recurrent endometrial cancer.
For Magellan’s clients, Ozempic had the highest trend of any product in 2022, and Wegovy was the fifth highest drug in terms of trend. Diabetes as a whole is expected to become the top condition leading pharmacy trend by 2024.
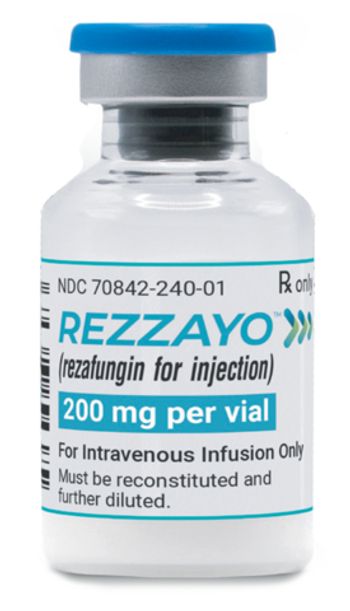
Rezzayo is a novel once-weekly antifungal to treat invasive candidiasis, a serious infection that can affect the blood, heart and brain. It will have a wholesale acquisition cost of $1,950 per vial.

The FDA is requiring Citius Pharmaceuticals to do enhanced product testing for Lymphir (formerly I/Ontak) to treat patients with cutaneous T-cell lymphoma.

One of the two recalled lots of the birth control tested high for a known impurity.

Harm Reduction Therapeutics will supply at least 200,000 free doses of RiVive to communities that need it most.

The supply of fentanyl, epinephrine injection, and heparin, however, have been impacted by the July 19 tornado at Pfizer's North Carolina facility.