
This is the second recall of Sandimmune related to the formulation of crystals in some bottles.

This is the second recall of Sandimmune related to the formulation of crystals in some bottles.

A recent report predicts there could be an increase in premiums for Medicare Part D prescription drug plans in California, Florida, New York, Pennsylvania and Texas.

A simulation study estimated the impact of biosimilar substitution on total cost of care and provider financial performance in the final performance period of the Oncology Care Model.

Newly launched include generics of Spiriva HandiHaler, Forteo, Livalo and Farxiga.

Janssen is seeking approval for use after Tagrisso in patients with metastatic non-small cell lung cancer.

A regulatory decision will not be made by the Prescription Drug User Fee Act (PDUFA) target action date of Dec. 16, 2023.

New biosimilars have been added and others removed from CVS Caremark’s standard formulary. One notable change is with the Humira biosimilars. The PBM has removed Amjevita and now prefers Hyrimoz and an unbranded biosimilar.

Truqap is an AKT kinase inhibitor that, along with Faslodex, reduced the risk of disease progression or death by 50%.

Contamination with Penicillium brevicompactum could lead to invasive fungal infections of the blood or pneumonia that can be life-threatening in immunocompromised patients.

Augtyro will be available in mid-December to treat patients with metastatic ROS1-positive non-small cell lung cancer. It has a list price of $29,000 a month.

This is the latest offering by Express Scripts that aims to bring transparency to prescription drug costs.

Bimzelx is the first dual IL-17 A/F inhibitor to treat moderate-to-severe plaque psoriasis. It launches with a list price of $7,200 per syringe.

The results of a recent feasibility study on the outpatient administration of cell therapies is creating growing interest in whether home-based management may be possible in the future.

Aliqopa was granted accelerated approval in September 2017 to treat adult patients with relapsed follicular lymphoma.


Ixchiq was approved through the FDA Accelerated Approval pathway.

Fruzaqla is the first inhibitor of all three VEGF receptor kinases. The list price is $25,200 for a 28-day supply of a 5 mg dose and $6,300 for a 28-day supply of 1 mg dose

Adzynma is a recombinant protein designed to replace the deficient ADAMTS13 enzyme.

Beginning Jan. 1, 2024, Cigna Healthcare’s formulary changes mostly impact generics and biosimilars.

In her review of the specialty drug pipeline, Evernorth's Aimee Tharaldson said upcoming approvals for Crohn’s and colitis drugs could further shift the drug spend from the medical benefit to the pharmacy one.
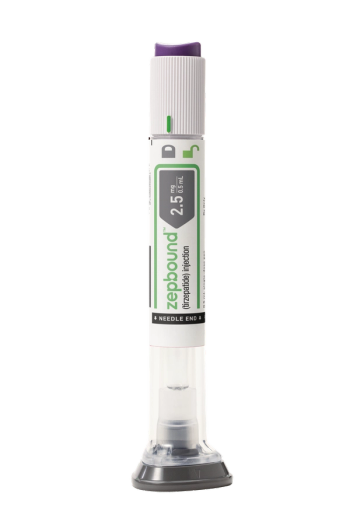
Tirzepatide, with the brand name Zepbound, is expected to be available by the end of the year in six doses at a list price of $1,059.87, which is about 20% lower than semaglutide.

Zurzuvae, the first oral therapy for postpartum depression, will launch in December.

Using an AI platform, Magellan Health was able to better support providers in prescribing behavioral health medications and addressing medication problems, which reduced pharmacy costs and increased adherence.

First in class, biosimilars, oncology drugs and even some generics have been added to Optum Rx’s list of exclusions for 2024.

The Institute for Clinical and Economic Review’s fair access report found that for the drugs assessed, formularies provided fair access, but it was difficult to determine how well that translates into real-world access and affordability for patients.