
Regulators will continue to evaluate a possible link by reviewing meta-analysis of clinical trials across all GLP-1 products and analyzing postmarketing data in the Sentinel System.

Regulators will continue to evaluate a possible link by reviewing meta-analysis of clinical trials across all GLP-1 products and analyzing postmarketing data in the Sentinel System.

Vevye is a water- and preservative-free solution of cyclosporine, which allows for improved bioavailability.

Clients are cautious about how the cost in “cost-plus” programs is determined, how rebates factor in and the benchmarks used, says Navitus’s Brent Eberle.

The transaction is valued at about $575 million. The sale is part of Rite Aid’s restructuring plan after filing for bankruptcy in October 2023.
Tivdak was granted accelerated approval in September 2021 to treat patients with recurrent or metastatic cervical cancer. The goal date for full approval is May 9, 2024.
The FDA cited issues with a third-party manufacturing company. Zolbetuximab is being reviewed to treat patients with stomach cancer.
Zelsuvmi, a first-in-class topical treatment approved for patients with molluscum contagiosum, will be available in the second half of 2024.

Adam Fein, founder of Drug Channels, said the acquisition will allow the company to upgrade its technology, grow the company and provide live and in-person events.

If approved, Accord’s biosimilar would treat several autoimmune conditions and would be launched no later than May 15, 2025

CVS Caremark has entered into an agreement with AbbVie, the manufacturer of Humira, to supply Cordavis with Humira to develop a cobranded product. Cordavis is a CVS company developing private label therapies.
Zilbrysq has a list price $1,047.19 for a 23 mg syringe. It is UCB’s second treatment for patients with myasthenia gravis.
Loqtorzi has a wholesale acquisition cost of $8,892.03 per single-use vial.
The presence of glass can lead to serious adverse events, including inflammation of a vein and blockage of blood vessels or life-threatening blood clots.






Wainua is approved to treat patients with hereditary transthyretin-mediated amyloid polyneuropathy and can be self-administered via an auto-injector.
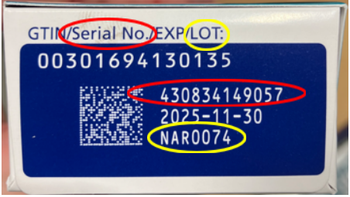
The FDA has seized thousands of units of counterfeit Ozempic 1 mg.

This is the first treatment for patients with primary immunoglobulin A nephropathy to be granted full approval.

This is the second complete response letter for gefapixant, and FDA officials said the application did not provide evidence of effectiveness.

Nonprescription RiVive was approved in July 2023 to reverse opioid overdose. Harm Reduction is donating 200 doses to Remedy Alliance for distribution.
Outlook Therapeutics plans to begin a study in the first quarter of 2024 to address the issues identified in a FDA complete response letter. If approved, Lytenava would be the only bevacizumab product to specifically treat age-related macular degeneration.
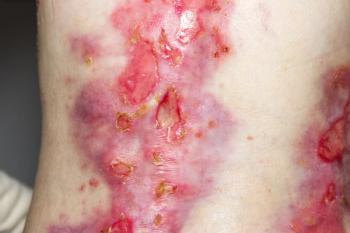
Filsuvez treats children and adults with epidermolysis bullosa, a condition that causes the skin to blister, and junctional epidermolysis bullosa, which causes lesions in the mouth.

In other generic news, the FDA has approved generics of the osteoporosis drug Forteo and Amneal is developing a generic version of Vascepa.