Biosimilars
Latest News
CME Content

Several biosimilars will come to market within the next few years.
Study finds that biologics that faced competition from biosimilars showed reductions in net prices and leveling off of list price increases.

The FDA Commissioner says that insurers can help biosimilar adoption by taking short-term financial losses over long-term drug savings. What else should they be doing?
The Biosimilars Forum, in partnership with Medicines for Europe, says the U.S. can look to European policy directives to advance the biosimilars market.
The falsehoods being spread about biosimilars that don’t match up with reality.
The FDA issued a lengthy statement on biosimilar naming conventions-but industry groups worry it could disincentivize biosimilar development.

Industry groups are calling for clear FDA guidance on language used by biologic originators to protect market share.

What the future of specialty drugs holds for healthcare.
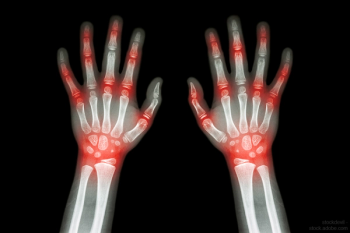
The latest in RA therapies, and what you can expect from the pipeline.
Biosimilar drugs are poised to transform the cancer drug market-so why is uptake so slow?

As more biosimilar agents are approved by the FDA, biologic manufacturers are looking for unique ways to hold on to market share.

While still largely a new phenomenon, biosimilars are here to stay-and this year will only bring more of them to market.
Biosimilar adoption is not happening as fast as once expected-will fears about future growth hold the market back?

The partial government shutdown is affecting the country in many ways-but how is it interfering with biosimilar development?

With a new ruling against the Affordable Care Act (ACA), many worry about the fate of the biosimilar market. But will it be as detrimental as feared?

2018 was meant to be a big year for the biosimilar market-but were developments as big as hoped? Find out.

In an effort to lower prescription drug prices, CMS unveiled proposed polices for 2020 to strengthen and modernize the Medicare Part C and D programs. Here’s a closer look at what CMS’ provisions entail and how they help accomplish the Administration’s four strategies.

With all eyes on drug prices, payers are looking to biosimilar agents to cut costs-and improve patient outcomes.

The biosimilar market is flourishing in Europe-why aren’t we seeing similar gains in the U.S.?

Despite a scolding from President Trump over drug price hikes, drug giant Pfizer announced it will raise the prices of 41 medications in January. That’s 10% of its entire drug portfolio.

Here are five things managed care executives should know about the proposed plan.

The value and benefits of biosimilars are increasingly becoming more apparent. Here are four areas where they are growing the most.

This comprehensive, pay-for-performance approach drives clinical quality and affordability.

Biosimilar agents can help healthcare organizations offer patients more options for their care. So, what’s stopping payers from immediately adding them to their formularies?