
Uptake of pre-exposure prophylaxis (PrEP) has been slow. A survey shows that safety and side effects are the top concerns.
Keith Loria is a contributing writer to Medical Economics.

Uptake of pre-exposure prophylaxis (PrEP) has been slow. A survey shows that safety and side effects are the top concerns.
Gastroesophageal reflux disease is a risk factor for what is now the more common type of esophageal cancer, adenocarcinoma. Immunotherapy is coming on strong as a treatment.
Tulane University researchers are studying whether dysregulation of the oral microbiome might affect HIV outcomes. They are using rhesus monkeys and SIV infection as a model.

The researchers have several suggestions for how harm from screening should be reported in screening guidelines.
Results of research by Swiss investigators show effector memory T cells bounce back quickly after autologous hematopoietic stem cell transplantation.
Florida has among the largest populations of people living with HIV in the country. We spoke with a clinician with 40 years of experience taking care of Floridians with HIV.
Research results published in Cell Reports implicate the Nef protein as a possible cause of disease-causing inflammation that persists even when an HIV infection is controlled.
HIV hides out and replicates in the spleen, the lymph nodes and other reservoirs. Emmanuel Okala hopes to develop nanotechnology that will flush it out.

University of Arkansas researchers added to the understanding of how anxiety affects sleep with research that looked for associations between anxiety and bedtime procrastination and other patterns of poor sleep.

Dana-Farber Cancer Institute tool evaluates the probability of having genetic mutations that confer elevated risk for 18 cancers.
Results of a small study published in Nature Medicine show that early treatment with a monoclonal antibody might be part of a strategy to clear an HIV infection.
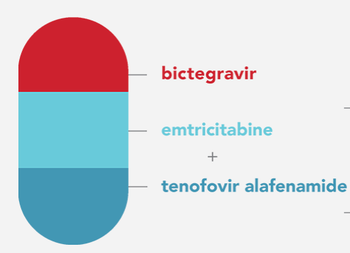
Gilead Sciences reported results from several studies of its antiretroviral.

In a conversation with Managed Healthcare Executive®, Genoa Healthcare Sales Director Jennifer Finocchiaro discusses social determinants of health, the LGBTQ+ community and the demographic contours of the HIV/AIDS epidemic.

Researchers at Harvard-affiliated Brigham and Women's Hospital identified common beliefs among parents and caregivers and also evidence showing they were wrong. They say that they hope this kind of research will lead to public outreach and education efforts to turn people away from misinformation.
Small study finds that sleep deprivation increases white blood cells. The finding is a piece of a puzzle that may link poor sleep to inflammation and a number of diseases.
Escalating costs are hitting patients hard. CMS price negotiation and the $2,000 cap on Part D out-of-pocket expenses should benefit many patients with Medicare coverage.

Escalating costs are hitting patients hard. CMS price negotiation and the $2,000 cap on Part D out-of-pocket expenses should benefit many patients with Medicare coverage.

About 40% of people monkeypox also had HIV, according to a CDC study that included almost 2,000 monkeypox cases in the U.S. that occurred during the first two months of the outbreak.
People with HIV are more susceptible to getting COVID-19 and having a severe case. Researchers at the University of Miami Miller of School of Medicine are heading up an NIAID-funded study that will use artificial intelligence to observe the overlap of the two infectious diseases and the evolution new variants of the SARS-CoV-2 virus that causes COVID-19.

Ryan White funds can make HIV care affordable, even free, but there needs to be more outreach to the trans community, says University of California, Irvine, professor.

Preexposure prophylaxis and emphasizing the U=U (undetectable = untransmittable) message are central to the efforts to end the HIV epidemic, a goal that the Biden administration picked up from the Trump administration.

ViiV Healthcare reports long-term positive results for its drug, Rukobia (fostemsavir).
More of these treatments have been approved, but supply chain issues and cost remain obstacles.

Schmid, a long-time leader in the HIV advocacy world and executive director of the HIV+Hepatitis Policy Institute, says a national program to promote and cover the costs of preexposure prophylaxis (PrEP) is desperately needed.

Brii Biosciences is one of the drug developers working on regimens that would free people living with HIV from daily pills.

UCLA research Beth D. Jamieson explores the relationship between HIV and aging. Recent evidence suggests that HIV may accelerate aging within two or three years of the initial infection.

Although the Oncology Care Model produced some positive results, the value-based care model cost Medicare money. Now attention is turning to its successor, the Enhancing Oncology Model.

The risk of breakthrough was higher in people living with HIV regardless of CD4 count or HIV viral suppression, according to findings reported by researchers at Johns Hopkins.

The new policy removes any restrictions on members of the armed forced who are HIV positive who are asymptomatic and have a viral load that is undetectable.