The bipartisan bill introduced in the Senate in May would require PBMs to report to the FTC how much money they make through spread pricing and pharmacy fees.

The bipartisan bill introduced in the Senate in May would require PBMs to report to the FTC how much money they make through spread pricing and pharmacy fees.
Next year, seven biosimilars of Humira are expected to launch, but leaders from the Biosimilars Forum are concerned not all will make it onto formularies.

The U.S. government pre-purchases 3.2 million initial doses of the Novavax COVID-19 vaccine.

Violaine Harris, Ph.D., a senior research scientist with Tisch MS Research Center of New York, discusses new research that shows stem cell treatments can improve disability in patients with MS.
Under the national vaccine strategy, the U.S. Department of Health and Human Services is expanding access to the monkeypox vaccine, Jynneos, in areas with the highest transmission and need.

The United States has acquired adult and pediatric doses of the COVID-19 vaccine for delivery in early fall in a contract worth $3.2 billion, as well as an additional 150,000 doses of bebtelovimab for about $275 million.

The FDA is accepting comments on the proposed rule through Oct. 26, 2022.

The trial was an observational study aimed at collecting real-world data on the Alzheimer’s treatment.

On June 15, 2022, the FDA’s Vaccines and Related Biological Products Advisory Committee unanimously voted in favor of both vaccines.

The committee recommended Moderna’s and Pfizer’s COVID-19 vaccines were recommended for children 6 months to five years, as well as Moderna’s vaccine for children ages 7 to 16.
Efanesoctocog alfa is a new class of factor VIII therapy with once-weekly prophylactic dosing. The application for approval expected to be filed mid year.

The recalls were made because the medications failed sterility testing.

Novavax’s COVID-19 vaccine demonstrated 90.4% efficacy with a low number of serious and severe adverse events, including myocarditis.

This is the first study to examine the effects of vibration training on changing cognition and quality of life in people with multiple sclerosis.
The lack of biomarkers for progressive multiple sclerosis has made the development of new treatments much more difficult for this form of the disease.

A study in mice has found that a combination immunotherapy slowed tumor growth and activated anti-tumor immune response.

Researchers stress that attention to cardiac risk intervention is warranted for lean patients with nonalcoholic fatty liver disease (NAFLD)

The Access to Oncology Medicines Coalition brings together pharma companies and other organizations to help countries develop the capacity and access to essential cancer medicines.

The FDA emergency use authorization allows the nonprescription test to use at-home sample collection with testing performed in a laboratory.

Mounjaro is a first-in-class medicine that activates both the GLP-1 and GIP receptors, which leads to improved blood sugar control.

Olumiant is the first immunomodulatory treatment for COVID-19 to receive FDA approval.

The move to limit Janssen’s vaccine comes after an analysis finds there is a risk of thrombocytopenia syndrome (TTS), a rare syndrome of blood clots and low levels of blood platelets.
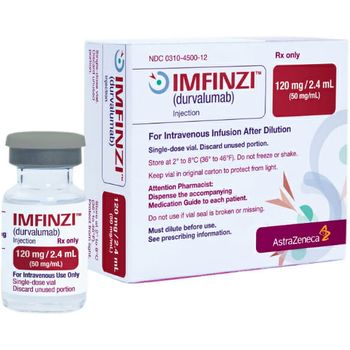
The FDA’s Prescription Drug User Fee Act action date for its regulatory decision for Imfinzi for BTC is during the third quarter of 2022.

Anthony Feinstein, Ph.D., of the University of Toronto, author of a new book about multiple sclerosis and mood, says clinicians and patients need to pay more attention to the effects that depression has on the cognitive abilities of patients with the disease.

Study in Czech Republic followed 392 patients for approximately four years.
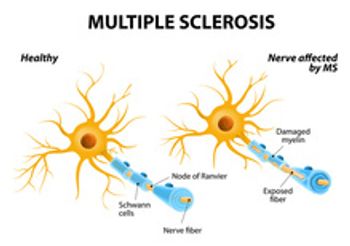
Blood-based biomarker test can be used to assess disease activity in patients with multiple sclerosis and for treatment response monitoring.

UnitedHealthcare considers Aduhelm unproven and not medically necessary for treating Alzheimer’s disease.
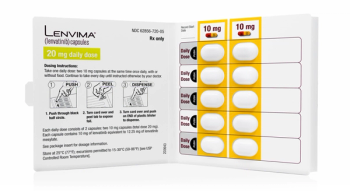
Lenvima is approved to treat thyroid cancer, renal cell carcinoma and in combination with Keytruda for metastatic kidney cancer.

An animal study of histotripsy suggests that powerful, focused ultrasound might be a way of treating liver cancer. It is a preliminary study. Much more research will need to be done before the technology is accepted as safe and effective.

The goal is to screen 20,000 people in the U.S. with at-home genetic tests for nonalcoholic fatty liver disease (NAFLD).