
The drug won't be available till next year but is expected to dent sales of Roche's blockbuster for age-related macular degeneration and other eye diseases.

The drug won't be available till next year but is expected to dent sales of Roche's blockbuster for age-related macular degeneration and other eye diseases.
The FDA has set a Prescription Drug User Fee Act target action date of April 30, 2022.

Jardiance demonstrated a 21% relative risk reduction for the composite primary endpoint of cardiovascular death or hospitalization for heart failure.

Officials with OptumRx predict four new therapies that are expected to an impact on patients and on the market.

Investigators find there is strong adherence soon after patients fill prescriptions but less consistent use after initial treatment.
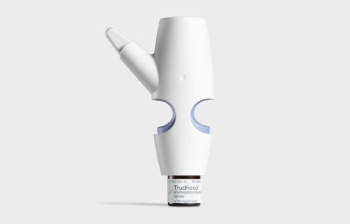
The therapy, to be launched in October, is delivered quickly to the bloodstream by targeting the upper nasal space.
A large Netherlands study demonstrated that rituximab biosimilars (R-biosimilars) produced a 43% reduction in annual costs. In addition, three-year overall survival did not differ between diffuse large B-cell lymphoma (DLBCL) and those receiving rituximab, according to the researchers.
Lack of antibodies from vaccination mainly affects patients with B cell cancers. Experts say antibodies are just one piece of the puzzle and that other aspects of the immune system fend off serious cases of COVID-19.

U.S. hospitals are spending $310 million annually to manage the additional clinical, operational, logistical, and patient care work associated with white and brown bagging requirements, according to a new report.

The FDA’s review of the JAK inhibitor class concluded there is an increased risk of serious heart-related events such as heart attack or stroke, cancer, blood clots, and death with these arthritis and ulcerative colitis medicines.
A targeted therapy using non-thermal radio waves improved overall survival in hepatocellular carcinoma (HCC) patients and had no side effects, a new study found.
Pharmacists are referring patients elsewhere or are shifting patients who were using the IV form to the subcutaneous injection. The NIH has also recommended using Kevzara as an alternative where there are shortages of Actemra.

Florida’s First Choice Neurology has provided Aduhelm to patients through the Biogen access program.

Preliminary research shows Anplag could be repurposed as a heart failure treatment at a lower cost.

Now called Comirnaty, the vaccine is approved for those 16 years and older.

The therapy is expected to be available by early September.

The VA recommends against offering this agent to patients with Alzheimer’s dementia, mild or otherwise.

The program and proposed integrations could produce $15 million in savings annually.

Libtayo also reduced the risk of death by 29% compared with chemotherapy alone.

The therapy can now be used for post-exposure prevention in people at high risk for progression to severe disease.
The FDA is expected to make a decision on this indication by December 1, 2021.

Coffee may have some protective properties. Alcohol implicated, again, as a risk factor.
Micronoma, a San Diego company, says its technology picks up signs of very early cancer from disrupted microbiome.

The agent targets excessive daytime sleepiness.
FDA approval means that pharmacists can swap biosimilar Semglee, an insulin product, for brand-name Lantus, although state-level pharmacy rules may apply.
Angiotensin receptor blockers may be less likely to cause side effects than ACE inhibitors.

Most of the suspected cases of myocarditis developed after the second dose of the vaccine and were among younger males.

This research sought to understand the relationship of prior medication exposure, existing health conditions, and COVID-19 outcomes using data from the American Heart Association’s COVID-19 Cardiovascular Disease Registry.

Research note from SVB Leerink says revenue erosion once biosimilars hit the market in 2023 may be more gradual than some prior predictions.

The new indication provides a subcutaneous option for multiple myeloma that can be administrated in minutes, rather than hours.