
The film version may be well-suited for many patients who have migraine-related nausea, says the president of the company that developed the medication.

The film version may be well-suited for many patients who have migraine-related nausea, says the president of the company that developed the medication.
The FDA cited issues related to the proposed manufacturing of the drug, mirikizumab. Lilly expressed confidence that they could be corrected.
Through Express Scripts’ new Copay Assurance plan, consumers will have lower out-of-pocket costs because of caps on prescription drugs: $5 for generics, $25 for preferred brand drugs, and $45 for preferred specialty brand drugs.

Other indications for the blockbuster cancer mediation are not affected.

This case and a case in Washington state are likely to be appealed to the Supreme Court.

Issued jointly by the FDA Commissioner and Chief Scientist, the decision means Makena and its generics are no longer approved and cannot lawfully be distributed in interstate commerce. At the same time, the agency “recognizes that there is a supply of product that has already been distributed.”

The combination of Padcev with Merck’s Keytruda has a potentially large market: approximately 8,000 to 9,000 U.S. patients would be eligible for this combination in the U.S.

State officials say the new NYRx program will mean fewer restrictions and create a largest pharmacy network in the state.

A bipartisan Senate bill, Affordable Insulin Now Act of 2023, would require plans to cover insulin for no more than $35 per month.
Emergent BioSolutions hasn’t provided a price for the nonprescription Narcan but said the product will be available by late summer.
Rezzayo is a novel once-weekly antifungal approved to treat invasive candidiasis, a serious infection that can affect the blood, heart and brain.

CalRx will work with Civica to provide the most commonly used short- and long-acting insulins at planned prices of no more than $30 a vial and $55 for a box of five pre-filled pens.

The company also will establish a $35 cap on out-of-pocket costs for Lantus for all patients with commercial insurance.

The lower prices will take effect on Jan. 1, 2024, on both pre-filled pens and vials of basal, bolus and pre-mix insulins, including Levemir, Novolin, NovoLog and NovoLog Mix 70/30.

Daybue will be available by the end of April with a list price of about $375,000 annually.

Other news includes the launches of the first generic of an ulcer drug, the antipsychotic lurasidone, an extended-release version of topiramate for migraine, and additional strengths of the cancer drug lenalidomide. Additionally, Glenmark will distribute Cediprof's generic Adderall, and the FDA has accepted Amneal’s application for a generic naloxone nasal spray.
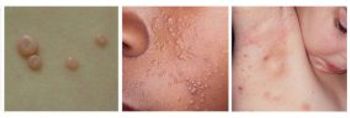
If approved, berdazimer could be the first prescription product treat molluscum, a common skin infection caused by poxvirus. The Prescription Drug User Fee Act goal date is Jan. 5, 2024.
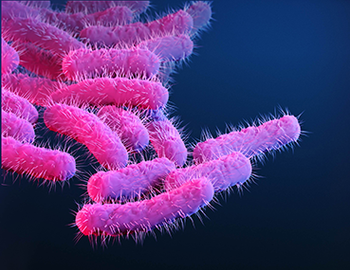
Shigella cause an estimated 450,000 infections in the United States each year and an estimated $93 million in direct medical costs.

The companies have also submitted an EUA for a fourth dose of the bivalent COVID-19 vaccine in children 6 months through 4 years of age.

The ads focus on how PBMs promote competition in the prescription drug marketplace and provide choice for employers.
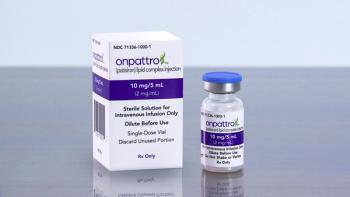
Alnylam is seeking approval for Onpattro’s use in cardiomyopathy related to transthyretin-mediated (ATTR) amyloidosis. The FDA set an action date of Oct. 8, 2023.

The U.S. public health emergency response to COVID-19 ends May 11, 2023, and the transition to more traditional healthcare coverage will begin later this year.

Hospira/Pfizer’s potassium phosphates product alone may produce daily aluminum exposures of up to twice of the FDA-recommended limit for children.
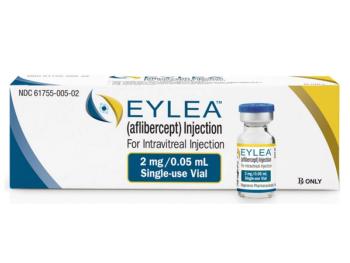
Eylea now also treats retinopathy of prematurity, a leading cause of childhood blindness worldwide.
Takhzyro is the first therapy to prevent attacks of hereditary angioedema (HAE) in children ages 2 to under 6.
The products may be ineffective, unsafe and could prevent a person from seeking an appropriate diagnosis.

Vizient is forecasting a 3.78% rise in pharmacy prices in hospitals and non-acute care facilities, reflecting increases in both prices and utilization.

Evio is launching a new pharmacy solution that leverages Flipt’s proprietary, cloud-based benefit administration and patient engagement platforms.

The Association for Accessible Medicines’ report indicates plans, especially those in Medicare, are slower to prefer first generics.

Moderna plans to submit to the FDA its investigational mRNA vaccine, RNA-1345, for respiratory syncytial virus (RSV) in the first half of 2023.